
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Europe

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
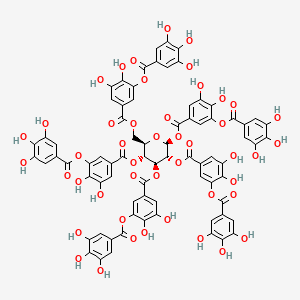

1. 1401-55-4
2. Glycerite
3. Gallotannin
4. Gallotannic Acid
5. 5424-20-4
6. Chinese Gallotannin
7. Mfcd00066397
8. Mls001335996
9. [2,3-dihydroxy-5-[[(2r,3r,4s,5r,6s)-3,4,5,6-tetrakis[[3,4-dihydroxy-5-(3,4,5-trihydroxybenzoyl)oxybenzoyl]oxy]oxan-2-yl]methoxycarbonyl]phenyl] 3,4,5-trihydroxybenzoate
10. Chebi:81066
11. Smr000857330
12. Dsstox_cid_6076
13. Dsstox_rid_78006
14. Dsstox_gsid_26076
15. [2,3-dihydroxy-5-[[3,4,5,6-tetrakis[[3,4-dihydroxy-5-(3,4,5-trihydroxybenzoyl)oxybenzoyl]oxy]oxan-2-yl]methoxycarbonyl]phenyl] 3,4,5-trihydroxybenzoate
16. Fema No. 3042
17. Quebracho Extract
18. Beta-d-glucose Pentakis(3,4-dihydroxy-5-((3,4,5-trihydroxybenzoyl)oxy)benzoate)
19. Beta-d-glucose Pentakis[3,4-dihydroxy-5-[(3,4,5-trihydroxybenzoyl)oxy]benzoate]
20. Cas-1401-55-4
21. Tannic-acid
22. Tannicum Acidum
23. Nsc656273
24. Nsc-656273
25. Nsc-758670
26. Ncgc00095101-01
27. Einecs 226-562-9
28. Tannin (tannic Acid)
29. Asian Holly Oak Nutgall
30. Tannic Acid, Unspecified
31. Tannic Acid, Acs Reagent
32. Tannic Acid, Technical Grade
33. Mls001335995
34. Schembl409692
35. Tannic Acid, Saj First Grade
36. Chembl506247
37. Gtpl4319
38. Bdbm60986
39. Dtxsid00892987
40. Tannic Acid, Puriss., 95.0%
41. Cid_16129778
42. Tox21_111422
43. Tox21_300079
44. Bdbm50442879
45. S3951
46. Akos015951319
47. Tannic Acid, Vetec(tm) Reagent Grade
48. Ccg-270692
49. Ncgc00186054-01
50. Ncgc00186054-02
51. Ncgc00253925-01
52. Tannic Acid, Tested According To Ph.eur.
53. C17409
54. Tannic Acid, Source: Chinese Natural Gall Nuts
55. A901485
56. Q427956
57. Q-201780
58. Tannic Acid, Puriss., Meets Analytical Specification Of Usp, Powder
59. Tannic Acid, United States Pharmacopeia (usp) Reference Standard
60. .beta.-d-glucopyranose, Pentakis[3,4-dihydroxy-5-[(3,4,5-trihydroxybenzoyl)oxy]benzoate]
61. Beta-d-glucopyranose Pentakis[3,4-dihydroxy-5-[(3,4,5-trihydroxybenzoyl)oxy]benzoate]
62. (2r,3r,4s,5r,6s)-4,5,6-tris({3,4-dihydroxy-5-[(3,4,5-trihydroxyphenyl)carbonyloxy]phenyl}carbonyloxy)-2-[({3,4-dihydroxy-5-[(3,4,5-trihydroxyphenyl)carbonyloxy]phenyl}carbonyloxy)methyl]oxan-3-yl 3,4-dihydroxy-5-[(3,4,5-trihydroxyphenyl)carbonyloxy]benzoate
63. (2s,3r,4s,5r,6r)-6-(((3,4-dihydroxy-5-((3,4,5-trihydroxybenzoyl)oxy)benzoyl)oxy)methyl)tetrahydro-2h-pyran-2,3,4,5-tetrayl Tetrakis(3,4-dihydroxy-5-((3,4,5-trihydroxybenzoyl)oxy)benzoate)
64. [2,3-dihydroxy-5-[[(2r,3r,4s,5r,6s)-3,4,5,6-tetrakis[[3,4-dihydroxy-5-(3,4,5-trihydroxybenzoyl)oxy-benzoyl]oxy]tetrahydropyran-2-yl]methoxycarbonyl]phenyl] 3,4,5-trihydroxybenzoate
| Molecular Weight | 1701.2 g/mol |
|---|---|
| Molecular Formula | C76H52O46 |
| XLogP3 | 6.2 |
| Hydrogen Bond Donor Count | 25 |
| Hydrogen Bond Acceptor Count | 46 |
| Rotatable Bond Count | 31 |
| Exact Mass | 1700.1729741 g/mol |
| Monoisotopic Mass | 1700.1729741 g/mol |
| Topological Polar Surface Area | 778 Ų |
| Heavy Atom Count | 122 |
| Formal Charge | 0 |
| Complexity | 3570 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
...FORMERLY USED ORALLY FOR SYMPTOMATIC TREATMENT OF DIARRHEA, TOPICALLY FOR MANAGEMENT OF EXTENSIVE BURNS, & RECTALLY FOR RELIEF OF VARIOUS RECTAL DISORDERS. ... USE...AS CHEMICAL ANTIDOTE IN POISONING IS ONLY OF LIMITED VALUE, & SOME METALS & ALKALOIDS ARE NOT PRECIPITATED BY IT. ...FEW IF ANY LEGITIMATE MEDICAL USES...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 951
IT OCCURS IN PRODUCTS FOR TREATMENT OF EFFECTS OF POISON-IVY & POISON-OAK & FOR OTHER SKIN APPLICATIONS.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V10 256
OINTMENT OR SPRAY OF TANNIC ACID IS USED IN TREATMENT OF BED SORES, WEEPING ULCERS, ETC. TANNIC ACID GLYCERITE NF XII WAS FORMERLY USED LOCALLY FOR SORE THROAT & STOMATITIS & TO HARDEN NIPPLES DURING NURSING.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 719
MEDICATION (VET): TOPICALLY, AS HEMOSTATIC ASTRINGENT ON MOIST ECZEMAS, GALLS, WOUNDS, & IN OTIS EXTERNA. ... USED IN REPOSITORY PARENTERAL VITAMIN B-12.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 583
For more Therapeutic Uses (Complete) data for TANNIC ACID (6 total), please visit the HSDB record page.
...INTERFERES WITH HIGHLY EFFICIENT ADSORBENT ACTION OF ACTIVATED CHARCOAL.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 951
...EMPLOYED IN TREATMENT OF BURNS. ... DISADVANTAGE OF TANNIC ACID IS THAT IT IS NOT ACTIVE GERMICIDE. IT IS ALSO ABSORBED FROM DENUDED SURFACES & MAY CAUSE SERIOUS SYSTEMIC TOXICITY, PARTICULARLY LIVER DAMAGE. FURTHERMORE, IT CAUSES NECROSIS OF VIABLE TISSUE IN BURNED AREA.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 719
VET: IT IS STOMACH IRRITANT. ... WARNING: EXCESSIVE DIETARY INTAKE CAN CAUSE GROWTH DEPRESSION & TOXICITY. USE IN ENEMAS MAY BE TOXIC.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 583
ERRATICALLY ABSORBED.
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-166
...ADMIN BY IP OR SC INJECTION TO RATS APPEARED IN LIVER 1 HR AFTER ADMIN & WAS CONCENTRATED IN NUCLEI AS EARLY AS 3 HR LATER.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V10 258
...INCR CONCN OF TANNIC ACID IN BLOOD OF RABBITS & DOGS GIVEN...BY STOMACH TUBE, WITH MAX LEVEL AFTER 3 HR. ABSORPTION...FROM COLON, AS SHOWN BY RISING BLOOD LEVELS, WAS DEMONSTRATED IN RABBITS, SHEEP, GOATS, RATS & DOGS.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V10 258
...SUFFICIENT TANNIC ACID MAY BE ABSORBED FROM GI TRACT, DENUDED SURFACES, & MUCOUS MEMBRANES TO CAUSE SEVERE CENTRALOBULAR NECROSIS OF LIVER.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 951
YIELDS GALLIC ACID IN RATS; BLUMENBERG, FW, & KESSLER, FJ, ARZNEIMITTEL-FORSCH, 10, 742 (1960). /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. T-1
TANNIC ACID IN TEA ACCOUNTS FOR USE OF STRONG TEA IN UNIVERSAL ANTIDOTE, PRESUMABLY FOR DUAL PURPOSE OF PRECIPITATING TOXIC ALKALOIDS & OF HARDENING SURFACE OF GI MUCOSA & ITS MUCOUS LAYER.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 719
Related Excipient Companies

Excipients by Applications
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
54
PharmaCompass offers a list of Tannins API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Tannins manufacturer or Tannins supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Tannins manufacturer or Tannins supplier.
PharmaCompass also assists you with knowing the Tannins API Price utilized in the formulation of products. Tannins API Price is not always fixed or binding as the Tannins Price is obtained through a variety of data sources. The Tannins Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tannins manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tannins, including repackagers and relabelers. The FDA regulates Tannins manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tannins API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Tannins manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Tannins supplier is an individual or a company that provides Tannins active pharmaceutical ingredient (API) or Tannins finished formulations upon request. The Tannins suppliers may include Tannins API manufacturers, exporters, distributors and traders.
click here to find a list of Tannins suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Tannins Drug Master File in Japan (Tannins JDMF) empowers Tannins API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Tannins JDMF during the approval evaluation for pharmaceutical products. At the time of Tannins JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Tannins suppliers with JDMF on PharmaCompass.
Tannins Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tannins GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tannins GMP manufacturer or Tannins GMP API supplier for your needs.
A Tannins CoA (Certificate of Analysis) is a formal document that attests to Tannins's compliance with Tannins specifications and serves as a tool for batch-level quality control.
Tannins CoA mostly includes findings from lab analyses of a specific batch. For each Tannins CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tannins may be tested according to a variety of international standards, such as European Pharmacopoeia (Tannins EP), Tannins JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tannins USP).
