
Synopsis
Synopsis
0
JDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
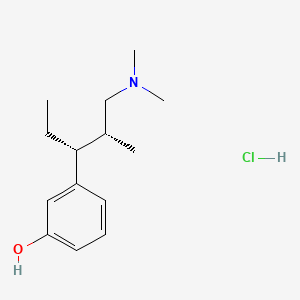

1. 3-((1r,2r)-3-(dimethylamino)-1-ethyl-2-methylpropyl)phenol
2. Nucynta
3. Tapentadol
1. 175591-09-0
2. Tapentadol Hcl
3. Nucynta
4. Palexia
5. Tapentadol (hydrochloride)
6. Nucynta Er
7. Bn-200 Hydrochloride
8. Cg5503 Hydrochloride
9. Cg-5503 Hydrochloride
10. 3-((2r,3r)-1-(dimethylamino)-2-methylpentan-3-yl)phenol Hydrochloride
11. 71204kii53
12. 175591-09-0 (hcl)
13. Tapentadol Hydrochloride (jan)
14. Tapentadol Hydrochloride [jan]
15. 3-[(1r,2r)-3-(dimethylamino)-1-ethyl-2-methylpropyl]phenol Hydrochloride
16. Palexia Retard
17. 3-((1r,2r)-3-(dimethylamino)-1-ethyl-2-methylpropyl)phenol Hydrochloride
18. Unii-71204kii53
19. Palexia Sr
20. Phenol, 3-[(1r,2r)-3-(dimethylamino)-1-ethyl-2-methylpropyl]-, Hydrochloride (1:1)
21. Phenol, 3-((1r,2r)-3-(dimethylamino)-1-ethyl-2-methylpropyl)-, Hydrochloride (1:1)
22. Nucynta (tn)
23. Schembl238138
24. Jns-024 Er
25. Chembl1201777
26. Dtxsid00938677
27. Amy25232
28. Hy-70042a
29. Mfcd00944992
30. Tapentadol Hydrochloride [mi]
31. Akos016842888
32. Cs-0879
33. Tapentadol Hydrochloride [mart.]
34. Tapentadol Hydrochloride [vandf]
35. Tapentadol Hydrochloride [who-dd]
36. Ac-32018
37. Tapentadol Hydrochloride [orange Book]
38. D10199
39. Tapentadol Hydrochloride [ep Monograph]
40. 591t238
41. Tapentadol Hydrochloride (1.0 Mg/ml In Methanol)
42. R-331333
43. Q27265914
44. 3-((1r,2r)-3-(dimethylamino)-1-ethyl-2-methylpropyl) Phenol Hydrochloride
45. 3-((1r,2r)-3-(dimethylamino)-1-ethyl-2-methylpropyl)-phenol Hydrochloride
46. 3-[(2r,3r)-1-(dimethylamino)-2-methylpentan-3-yl]phenol;hydrochloride
47. 3-[1-(dimethylamino)-2-methylpentan-3-yl]phenol--hydrogen Chloride (1/1)
48. (-)-(1r,2r)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol Hydrochloride
49. (-)-(1r,2r)-3-(3-dimethylamino-1-ethyl-2-methylpropyl)-phenol Hydrochloride
50. 3-[(1r,2r)-3-(dimethylamino)-1-ethyl-2-methylpropyl]phenol Monohydrochloride
51. Phenol, 3-((1r,2r)-3-(dimethylamino)-1-ethyl-2-methylpropyl)-, Hydrochloride
52. Phenol, 3-(3-(dimethylamino)-1-ethyl-2-methylpropyl)-, Hydrochloride, (r-(r*,r*))-
53. Tapentadol Hydrochloride Solution, 1.0 Mg/ml In Methanol (as Free Base), Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 257.80 g/mol |
|---|---|
| Molecular Formula | C14H24ClNO |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 5 |
| Exact Mass | 257.1546421 g/mol |
| Monoisotopic Mass | 257.1546421 g/mol |
| Topological Polar Surface Area | 23.5 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 193 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Nucynta |
| PubMed Health | Tapentadol (By mouth) |
| Drug Classes | Analgesic |
| Drug Label | NUCYNTA ER (tapentadol) is a mu-opioid receptor agonist, supplied in extended-release film-coated tablets for oral administration, containing 58.24, 116.48, 174.72, 232.96, and 291.20 mg of tapentadol hydrochloride in each tablet strength, correspo... |
| Active Ingredient | Tapentadol hydrochloride |
| Dosage Form | Tablet; Solution |
| Route | Oral |
| Strength | eq 100mg base; eq 50mg base; eq 75mg base; eq 20mg base/ml |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Nucynta er |
| Drug Label | NUCYNTA ER (tapentadol) is a mu-opioid receptor agonist, supplied in extended-release film-coated tablets for oral administration, containing 58.24, 116.48, 174.72, 232.96, and 291.20 mg of tapentadol hydrochloride in each tablet strength, correspo... |
| Active Ingredient | Tapentadol hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | eq 100mg base; eq 50mg base; eq 250mg base; eq 150mg base; eq 200mg base |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Nucynta |
| PubMed Health | Tapentadol (By mouth) |
| Drug Classes | Analgesic |
| Drug Label | NUCYNTA ER (tapentadol) is a mu-opioid receptor agonist, supplied in extended-release film-coated tablets for oral administration, containing 58.24, 116.48, 174.72, 232.96, and 291.20 mg of tapentadol hydrochloride in each tablet strength, correspo... |
| Active Ingredient | Tapentadol hydrochloride |
| Dosage Form | Tablet; Solution |
| Route | Oral |
| Strength | eq 100mg base; eq 50mg base; eq 75mg base; eq 20mg base/ml |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Nucynta er |
| Drug Label | NUCYNTA ER (tapentadol) is a mu-opioid receptor agonist, supplied in extended-release film-coated tablets for oral administration, containing 58.24, 116.48, 174.72, 232.96, and 291.20 mg of tapentadol hydrochloride in each tablet strength, correspo... |
| Active Ingredient | Tapentadol hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | eq 100mg base; eq 50mg base; eq 250mg base; eq 150mg base; eq 200mg base |
| Market Status | Prescription |
| Company | Janssen Pharms |
Treatment of acute pain
Treatment of acute pain
Treatment of acute pain
Treatment of chronic pain
Treatment of chronic pain
Treatment of acute pain
Treatment of chronic pain
Adrenergic Uptake Inhibitors
Drugs that block the transport of adrenergic transmitters into axon terminals or into storage vesicles within terminals. The tricyclic antidepressants (ANTIDEPRESSIVE AGENTS, TRICYCLIC) and amphetamines are among the therapeutically important drugs that may act via inhibition of adrenergic transport. Many of these drugs also block transport of serotonin. (See all compounds classified as Adrenergic Uptake Inhibitors.)
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)

Certificate Number : R0-CEP 2021-290 - Rev 00
Issue Date : 2022-11-24
Type : Chemical
Substance Number : 3035
Status : Valid

Date of Issue : 2022-08-11
Valid Till : 2025-06-26
Written Confirmation Number : WC-0054
Address of the Firm :
 Noramco is a partner to the pharmaceutical industry for controlled substance development & manufacturing.
Noramco is a partner to the pharmaceutical industry for controlled substance development & manufacturing.

GDUFA
DMF Review : Reviewed
Rev. Date : 2020-08-05
Pay. Date : 2020-07-30
DMF Number : 21084
Submission : 2008-01-23
Status : Active
Type : II

GDUFA
DMF Review : Reviewed
Rev. Date : 2013-03-13
Pay. Date : 2012-11-02
DMF Number : 26490
Submission : 2012-09-27
Status : Active
Type : II

NDC Package Code : 17180-9780
Start Marketing Date : 2012-06-26
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2013-03-01
Pay. Date : 2012-11-08
DMF Number : 26519
Submission : 2012-10-23
Status : Active
Type : II

Date of Issue : 2022-09-30
Valid Till : 2025-06-05
Written Confirmation Number : WC-0370
Address of the Firm :


GDUFA
DMF Review : Reviewed
Rev. Date : 2017-08-18
Pay. Date : 2017-03-30
DMF Number : 31435
Submission : 2017-03-27
Status : Active
Type : II
Date of Issue : 2019-11-18
Valid Till : 2022-06-05
Written Confirmation Number : WC-037a1
Address of the Firm :


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 28541
Submission : 2014-09-25
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Noramco is a partner to the pharmaceutical industry for controlled substance development & manufacturing.
Noramco is a partner to the pharmaceutical industry for controlled substance development & manufacturing.
GDUFA
DMF Review : Complete
Rev. Date : 2020-08-05
Pay. Date : 2020-07-30
DMF Number : 21084
Submission : 2008-01-23
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2013-03-01
Pay. Date : 2012-11-08
DMF Number : 26519
Submission : 2012-10-23
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2017-08-18
Pay. Date : 2017-03-30
DMF Number : 31435
Submission : 2017-03-27
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 28541
Submission : 2014-09-25
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-03-13
Pay. Date : 2012-11-02
DMF Number : 26490
Submission : 2012-09-27
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Certificate Number : R0-CEP 2021-290 - Rev 00
Status : Valid
Issue Date : 2022-11-24
Type : Chemical
Substance Number : 3035
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2021-390 - Rev 00
Status : Valid
Issue Date : 2022-10-03
Type : Chemical
Substance Number : 3035

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Tapentadol hydrochloride, Form-A
Certificate Number : CEP 2022-252 - Rev 00
Status : Valid
Issue Date : 2024-02-27
Type : Chemical
Substance Number : 3035

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] 
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
(2S, 3R)-1-(Dimethylamino)-3-(3-methoxyphenyl)-2-m...
CAS Number : 809282-20-0
End Use API : Tapentadol
About The Company : Cohance Lifesciences is a leading CDMO and API platform, offering products and services across all phases of a molecule’s lifecycle from development to commer...
CAS Number : 37951-49-8
End Use API : Tapentadol
About The Company : Cohance Lifesciences is a leading CDMO and API platform, offering products and services across all phases of a molecule’s lifecycle from development to commer...
CAS Number : 37951-49-8
End Use API : Tapentadol
About The Company : Sanika Chemicals Pvt. Ltd. is the fast growing company establishing a strong presence in the Domestic as well Global market as a reliable partner. We are very m...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?