
1. Barium, Tartrazine
2. Fd And C Yellow No. 5
3. Tartrazine Barium
4. Tartrazine Barium (2:3)
1. 1934-21-0
2. Acid Yellow 23
3. Yellow 5
4. Food Yellow 4
5. Aizen Tartrazine
6. Fd & C Yellow No. 5
7. Fd&c Yellow No. 5
8. 1342-47-8
9. Trisodium Salt
10. C.i. Acid Yellow 23
11. A.f. Yellow No. 4
12. Tartrazine Fd&c Yellow #5
13. Tartraphenine
14. Atul Tartrazine
15. Erio Tartrazine
16. Kako Tartrazine
17. Tartran Yellow
18. Tartrazine Lake
19. Tartrazine B
20. Tartrazine C
21. Tartrazine G
22. Tartrazine M
23. Tartrazine N
24. Tartrazine O
25. Tartrazine T
26. Ci 19140
27. Hd Tartrazine
28. Hydrazine Yellow
29. Lake Yellow
30. Sugai Tartrazine
31. Tartrazine Fq
32. Tartrazine Ns
33. Tartrazine Xx
34. Tartrazol Yellow
35. Wool Yellow
36. Tartrazine Xxx
37. Tartrazine Mcgl
38. Tartrazol Bpc
39. Amacid Yellow T
40. Cilefa Yellow T
41. Dye Yellow Lake
42. Fenazo Yellow T
43. Kayaku Tartrazine
44. Mitsui Tartrazine
45. Oxanal Yellow T
46. Tartar Yellow N
47. Tartar Yellow S
48. Tartrazine Yellow
49. Kiton Yellow T
50. Lemon Yellow A
51. Acid Yellow T
52. Bucacid Tartrazine
53. Dolkwal Tartrazine
54. Hexacol Tartrazine
55. Hidazid Tartrazine
56. Acilan Yellow Gg
57. C.i. 19140
58. Egg Yellow A
59. San-ei Tartrazine
60. Tartar Yellow Fs
61. Tartar Yellow Pf
62. Airedale Yellow T
63. Canacert Tartrazine
64. Food Yellow 5
65. Neklacid Yellow T
66. Tartrine Yellow O
67. Eurocert Tartrazine
68. Vondacid Tartrazine
69. Hydroxine Yellow L
70. Tartrazine C Extra
71. Calcocid Yellow Xx
72. Kca Tartrazine Pf
73. Yellow Lake 69
74. Naphtocard Yellow O
75. Tartrazine A Export
76. Hd Tartrazine Supra
77. Calcocid Yellow Mcg
78. Tartrazine Yellow 5
79. Yellow No. 5
80. Tartrazine A Expo T
81. Tartrazine B.p.c.
82. 12225-21-7
83. D&c Yellow 5
84. Food Yellow No. 4
85. Lemon Yellow A Geigy
86. Schultz No. 737
87. Maple Tartrazol Yellow
88. Acid Leather Yellow T
89. C.i. 640
90. Unitertracid Yellow Te
91. Yellow No. 5 Fdc
92. Curon Fast Yellow 5g
93. Fd And C Yellow 5
94. 1310 Yellow
95. 1409 Yellow
96. Xylene Fast Yellow Gt
97. Hispacid Fast Yellow T
98. Usacert Yellow No. 5
99. C.i. Food Yellow 4
100. Hexacert Yellow No. 5
101. Chebi:9405
102. Fd&g Yellow No. 5
103. Tartrazine Extra Pure A
104. L Yellow Z 1020
105. Tartrazine Lake Yellow N
106. Edicol Supra Tartrazine N
107. D And C Yellow No. 5
108. Kca Foodcol Tartrazine Pf
109. Certicol Tartrazol Yellow S
110. Tartrazine O Specially Pure
111. Tartrazine Xx Specially Pure
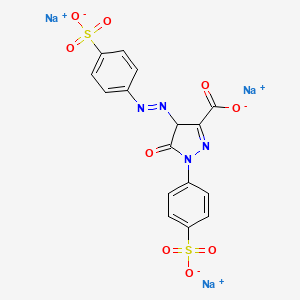
112. Trisodium 5-oxo-1-(4-sulfonatophenyl)-4-(4-sulfonatophenyl)diazenyl-4h-pyrazole-3-carboxylate
113. Dye Fd And C Yellow No. 5
114. Tartrazine Fd & C Yellow #5
115. E102
116. Kayaku Food Colour Yellow No. 4
117. C.i. Acid Yellow 23, Trisodium Salt
118. Trisodium;5-oxo-1-(4-sulfonatophenyl)-4-[(4-sulfonatophenyl)diazenyl]-4h-pyrazole-3-carboxylate
119. M-8847
120. Nsc4760
121. Zlut Kysela 23
122. 4,5-dihydro-5-oxo-1-(4-sulfophenyl)-4-((4-sulfophenyl)azo)-1h-pyrazole-3-carboxylic Acid, Trisodium Salt
123. Trisodium 5-oxo-1-(4-sulfonatophenyl)-4-[(e)-(4-sulfonatophenyl)diazenyl]-4,5-dihydro-1h-pyrazole-3-carboxylate
124. Zlut Pigment 100
125. Zlut Potravinarska 4
126. 3-carboxy-5-hydroxy-1-p-sulfophenyl-4-p-sulfophenylazopyrazole Trisodium Salt
127. L-gelb 2
128. L-gelb 2 [german]
129. Zlut Kysela 23 [czech]
130. Unii-i753wb2f1m
131. Zlut Pigment 100 [czech]
132. Zlut Potravinarska 4 [czech]
133. Tri Sodium Salt
134. Ccris 2656
135. Hsdb 7216
136. Fd & C Yellow No. 5 Tartrazine
137. Nsc 4760
138. Trisodium;5-oxo-1-(4-sulfonatophenyl)-4-[(e)-(4-sulfonatophenyl)diazenyl]-4h-pyrazole-3-carboxylate
139. Food Yellow No.4
140. Einecs 217-699-5
141. E 102
142. Epitope Id:124945
143. Tartrazine, Analytical Standard
144. Acid Yellow 23 Aluminium Lake
145. Tartrazine, P.a., 95-105%
146. Tartrazine, Dye Content >=85 %
147. Amy22425
148. Trisodium Salt Of 3-carboxy-5-hydroxy-1-sulfophenylazopyrazole
149. Tartrazine 100 Microg/ml In Water
150. Tartrazine, For Microscopy (hist.)
151. 1-(4-sulfophenyl)-4-((4-sulfophenyl)azo)-1h-pyrazole-3-carboxylic
152. Mfcd00148908
153. Akos015903034
154. Akos016010270
155. Trisodium 3-carboxy-5-hydroxy-1-p-sulfophenyl-4-p-sulfophenylazopyrazole
156. Trisodium 5-hydroxy-1-(4-sulphophenyl)-4-(4-sulphophenylazo)pyrazole-3-carboxylate
157. Bp-31013
158. Trisodium 4,5-dihydro-5-oxo-1-(4-sulfophenyl)-4-((4-sulfophenyl)azo)-1h-pyrazole-3-carboxylate
159. Trisodium 5-oxo-1-(4-sulfonatophenyl)-4-[(e)-(4-sulfonatophenyl)d Iazenyl]-4,5-dihydro-1h-pyrazole-3-carboxylate
160. Y-4
161. Ft-0621860
162. C.i. Acid Yellow 23, Trisodium Salt (van)
163. C07574
164. D90635
165. Q407158
166. W-107716
167. Tartrazine Lake, Ci No 19140:1 , Fd & C Yellow 5 Lake
168. 4,5-dihydro-5-oxo-1-(4-sulfophenyl)-4-((4-sulfophenyl)azo)-1h-pyrazole-3-carboxylic Acid
169. 5-oxo-1-(4-sulfophenyl)-4-[(e)-(4-sulfophenyl)azo]-4h-pyrazole-3-carboxylic Acid
170. 1h-pyrazole-3-carboxylic Acid, 4,5-dihydro-5-oxo-1-(4-sulfophenyl)-4-(2-(4-sulfophenyl)diazenyl)-, Sodium Salt (1:3)
171. 4,5-dihydro-5-oxo-1-(4-sulfophenyl)-4-(4-sulfophenyl)azo-1h-pyrazole-3-carboxylic Acid Trisodium Salt
172. Pyrazole-3-carboxylic Acid, 5-hydroxy-1-(p-sulfophenyl)-4-(p-sulfophenyl)azo-, Trisodium Salt
173. Sodium (e)-5-oxo-1-(4-sulfonatophenyl)-4-((4-sulfonatophenyl)diazenyl)-4,5-dihydro-1h-pyrazole-3-carboxylate
| Molecular Weight | 534.4 g/mol |
|---|---|
| Molecular Formula | C16H9N4Na3O9S2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 3 |
| Exact Mass | 533.95040307 g/mol |
| Monoisotopic Mass | 533.95040307 g/mol |
| Topological Polar Surface Area | 229 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 949 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
Food Coloring Agents
Natural or synthetic dyes used as coloring agents in processed foods. (See all compounds classified as Food Coloring Agents.)
Coloring Agents
Chemicals and substances that impart color including soluble dyes and insoluble pigments. They are used in INKS; PAINTS; and as INDICATORS AND REAGENTS. (See all compounds classified as Coloring Agents.)
... The fate of the pyrazole fragment of Tartrazine /was examined/ using sulphur-35 labelled Tartrazine and 1-(4-sulphophenyl)-3-methyl-4-(4-sulphophenylazo)-5-pyrazolone (SPMP an analogue of Tartrazine) and carbon-14 labeled SPMP. Following oral administration, both Tartrazine and SPMP labeled with sulphur-35 were predominantly excreted in feces (90 and 89 % of the dose respectively after 72 hours) with small amounts in urine (8 and 7.2 % of the dose, respectively, after 72 hours). The urinary radioactivity excreted in 48 hours with sulphanilic acid and 4-sulphophenylhydrazine was 23 and 23 % after Tartrazine administration, and 54 and 22 % after SPMP administration; the remaining radioactivity was not characterized.
European Food Safety Authority (EFSA); Scientific Opinion of the ANS Panel on the re-evaluation Tartrazine (E 102) Published: 12 November 2009 Available from, as of June 28, 2011: https://www.efsa.europa.eu/en/publications.htm
... The metabolism of carbon-14 Tartrazine randomly labeled in the phenyl azo group /was studied/ in rat, rabbit and human. In both animal species Tartrazine was administered orally and intraperitoneally whilst humans received oral Tartrazine. ... After intraperitoneal administration to 6 rats of 2.4 mg/kg bw of Tartrazine, between 64 and 96 % of the dose was recovered unchanged in urine within 24 hours; no other products were reported. In rabbit, at a dose of 2.4 mg/kg bw of Tartrazine administered intraperitoneally, 94 % of the dose was recovered unchanged in urine within 24 hours, with a further 1.4 % recovered as conjugated sulphanilic acid. However, after an intraperitoneal dose of 1000 mg in the rabbit ... only 57.3% was recovered unchanged in urine within 24 hours, with a further 25.7 and 6 % recovered as free and conjugated sulphanilic acid, respectively. After oral administration to 3 rats at 5 mg/rat ..., no free Tartrazine was measured but means of 28 and 34.6 % were recovered in urine as free and conjugated sulphanilic acid, respectively. In the rabbit dosed 1000 mg ... 8.2 % was recovered unchanged in urine within 24 hours with a further 27 and 26.8 % as free and conjugated sulphanilic acid respectively within 72 hours. In 4 humans receiving a single capsule containing 89-100 mg of Tartrazine ..., no free Tartrazine was measured in urine for any subject; in one subject 106 % was recovered as free sulphanilic acid whilst for the other 3 subjects mean recoveries of free and conjugated sulphanilic acid were 40.6 and 49.7 % respectively. ...
European Food Safety Authority (EFSA); Scientific Opinion of the ANS Panel on the re-evaluation Tartrazine (E 102) Published: 12 November 2009 Available from, as of June 28, 2011: https://www.efsa.europa.eu/en/publications.htm
Low biliary excretion of Tartrazine (1 %) /was demonstrated/ following intravenous administration of an unspecified dose. ... low biliary excretion was due to the carboxyl group. After a dose of 2 mg ... unchanged Tartrazine could be detected in bile, but there was no evidence of ring fission products. Following intraperitoneal injection, an unidentified and unquantified Tartrazine conjugate was rapidly excreted in bile, but again none of the previously reported reductive ring fission products.
European Food Safety Authority (EFSA); Scientific Opinion of the ANS Panel on the re-evaluation Tartrazine (E 102) Published: 12 November 2009 Available from, as of June 28, 2011: https://www.efsa.europa.eu/en/publications.htm
After oral administration there is extensive metabolism of Tartrazine by the gastrointestinal microflora to sulphanilic acid and aminopyrazalone (which may then be subsequently cleaved to sulphanilic acid and alpha-amino-beta-ketobutyric acid fragments with the latter breaking down further via intermediary metabolism with release of carbon dioxide).
European Food Safety Authority (EFSA); Scientific Opinion of the ANS Panel on the re-evaluation Tartrazine (E 102) Published: 12 November 2009 Available from, as of June 28, 2011: https://www.efsa.europa.eu/en/publications.htm
Absorption and metabolism of (14)C-labelled tartrazine (FD & C Yellow No. 5) and high molecular weight polymeric derivatives were compared in rats. A trace to 1.5% of unchanged monomeric dyes was excreted in urine and bile during the first 24 hr after dosing. No unchanged dye was absorbed after administration of the polymeric derivatives. ...In animals dosed with tartrazine and its polymer derivative, absorption of the cleavage product aminopyrazolone and its metabolites was 4.0 and 4.6%, respectively. Azo bond cleavage did not appear to be decreased in the polymer derivatives. However, the sulphanilic acid moiety of both dyes remained attached to the polymer backbone, resulting in a 95% decrease in sulphanilic acid absorption with polymeric tartrazine.
PMID:602250 Honohan T et al; Xenobiotica 7 (12): 765-774 (1977)
The 4-sulphophenylhydrazine metabolite was also labeled with sulphur-35 and administered orally and intraperitoneally. Excretion of this metabolite differed with the route of administration (35 and 49 % in urine and feces, respectively, 48 hours following oral, and 90 and 5 % in urine and feces, respectively, 48 hours following intraperitoneal administration). Following oral administration, 69 % of urinary radioactivity excreted in 48 hours was sulphanilic acid and 21 % was 4-sulphophenylhydrazine, whereas following intraperitoneal administration, 9 % of urinary radioactivity excreted in 48 hours was sulphanilic acid and 73% was 4-sulphophenylhydrazine. These data suggest there is a marked conversion of 4- sulphophenylhydrazine to sulphanilic acid presumably in the gut lumen. /4-sulphophenylhydrazine/
European Food Safety Authority (EFSA); Scientific Opinion of the ANS Panel on the re-evaluation Tartrazine (E 102) Published: 12 November 2009 Available from, as of June 28, 2011: https://www.efsa.europa.eu/en/publications.htm
... Blocking studies showed that tartrazine contraction was inhibited by atropine alone but not by any other blocking agent tested, implying that tartrazine acts either directly or indirectly upon the muscarinic acetylcholine receptor associated with parasympathetic innervation.
PMID:1570631 Hutchinson AP et al; Toxicol Lett 60 (2): 165-73 (1992)
The ability of selected food colors to interact with isolated guinea-pig ileum was investigated using a gut bath system. Studies revealed that guinea-pig ileum was specifically sensitive to tartrazine. Intestinal contraction occurred dose-dependently down to a minimum effective dose of 10 uM. ...Studies investigating the biological activity of structural analogues of tartrazine revealed the ability to initiate intestinal contraction was associated with the presence of the carboxylic acid residue at the R1 position of the pyrazole ring. Blocking studies showed that tartrazine contraction was inhibited by atropine alone but not by any other blocking agent tested, implying that tartrazine acts either directly or indirectly upon the muscarinic acetylcholine receptor associated with parasympathetic innervation.
PMID:1570631 Hutchinson AP et al; Toxicol Lett 60 (2): 165-73 (1992)