



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
EU WC
Listed Suppliers

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
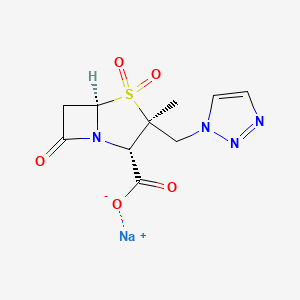

1. Tazobactam
2. Ytr 830
3. Ytr 830h
4. Ytr-830
5. Ytr830
1. Tazobactam Sodium Salt
2. 89785-84-2
3. Tazobactum Sodium
4. Tazobactam Sodium [usan]
5. Cl-307579
6. Tazobactam (sodium)
7. Ytr-830
8. Tazobactam (as Sodium)
9. Cl 307,579
10. Uxa545abtt
11. Chembl1439
12. Cl 307579
13. Chebi:85192
14. 89785-84-2 (sodium)
15. Ytr 830
16. 2-alpha-methyl-2-beta-(1,2,3-triazol-1-ylmethyl)penam-3-alpha-carboxylic Acid Sodium Salt
17. Dsstox_cid_26030
18. Dsstox_rid_81299
19. Dsstox_gsid_46030
20. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 3-methyl-7-oxo-3-(1h-1,2,3-triazol-1-ylmethyl)-, 4,4-dioxide, Sodium Salt, (2s,3s,5r)-
21. Sodium (2s,3s,5r)-3-methyl-7-oxo-3-(1h-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylate, 4,4-dioxide
22. Sodium;(2s,3s,5r)-3-methyl-4,4,7-trioxo-3-(triazol-1-ylmethyl)-4lambda6-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
23. Cas-89785-84-2
24. Unii-uxa545abtt
25. Tazobactum
26. Ncgc00159340-02
27. Schembl259991
28. Tazobactam Sodium (jan/usan)
29. Tazobactam Sodium [jan]
30. Dtxsid8046030
31. Tazobactam Sodium [vandf]
32. Tazobactam Sodium [mart.]
33. Yp-14
34. Tazobactam Sodium [who-dd]
35. Sodium (2s,3s,5r)-3-((1h-1,2,3-triazol-1-yl)methyl)-3-methyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate 4,4-dioxide
36. Tazobactam Sodium Salt [mi]
37. Tox21_111584
38. Bdbm50157692
39. Mfcd00917472
40. Akos015962921
41. Akos016843926
42. Tox21_111584_1
43. Cs-w009884
44. Hy-w009168
45. Tazobactam Sodium [orange Book]
46. Zosyn Component Tazobactam Sodium
47. Ncgc00159340-05
48. Ac-18978
49. Ac-32478
50. As-56873
51. Zerbaxa Component Tazobactam Sodium
52. Tazobactam Sodium Salt, Analytical Standard
53. Tazobactam Sodium Component Of Zosyn
54. Tazobactam Sodium Component Of Zerbaxa
55. D03707
56. T-1446
57. Tazobactam Sodium Salt, Beta-lactamase Inhibitor
58. Q27158392
59. Tazobactam Sodium Salt, Antibiotic For Culture Media Use Only
60. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 3-methyl-7-oxo-3-(1h-1,2,3-triazol-1-ylmethyl)-, 4,4-dioxide, Sodium Salt, (2s-(2alpha,3beta,5alpha))-
61. Sodium (2s,3s,5r)-3-methyl-4,4,7-trioxo-3-[(1h-1,2,3-triazol-1-yl)methyl]-4lambda(6)-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
62. Sodium (2s,3s,5r)-3-methyl-4,4,7-trioxo-3-[1,2,3]triazol-1-ylmethyl-4lambda6-thia-1-aza-bicyclo[3.2.0]heptane-2-carboxylate
63. Sodium(2s,3s,5r)-3-((1h-1,2,3-triazol-1-yl)methyl)-3-methyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate4,4-dioxide
64. Sodium; (2s,3s,5r)-3-methyl-4,4,7-trioxo-3-[1,2,3]triazol-1-ylmethyl-4lambda*6*-thia-1-aza-bicyclo[3.2.0]heptane-2-carboxylate
| Molecular Weight | 322.28 g/mol |
|---|---|
| Molecular Formula | C10H11N4NaO5S |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Exact Mass | 322.03478492 g/mol |
| Monoisotopic Mass | 322.03478492 g/mol |
| Topological Polar Surface Area | 134 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 579 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
beta-Lactamase Inhibitors
Endogenous substances and drugs that inhibit or block the activity of BETA-LACTAMASES. (See all compounds classified as beta-Lactamase Inhibitors.)




