
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Weekly News Recap #Phispers
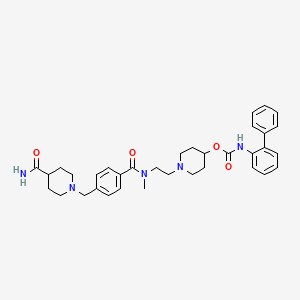

1. Biphenyl-2-ylcarbamic Acid 1-(2-((4-(4-carbamoylpiperidin-1-ylmethyl)benzoyl)methylamino)ethyl)piperidin-4-yl Ester
2. Td-4208
3. Yupelri
1. 864750-70-9
2. Td-4208
3. Yupelri
4. Gsk-1160724
5. Gsk1160724
6. Revefenacin [inn]
7. Revefenacin [who-dd]
8. G2ae2ve07o
9. 864750-70-9 (free Base)
10. [1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate
11. 1-(2-(4-((4-carbamoylpiperidin-1-yl)methyl)-n-methylbenzamido)ethyl)piperidin-4-yl N-((1,1'-biphenyl)-2-yl)carbamate
12. 1-(2-(3-((4-carbamoylpiperidin-1-yl)methyl)-n-methylbenzamido)ethyl)piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate
13. Biphenyl-2-ylcarbamic Acid, 1-(2-((4-(4-carbamoylpiperidin-1-ylmethyl)benzoyl)methylamino)ethyl)piperidin-4-yl Ester
14. Carbamic Acid, N-(1,1'-biphenyl)-2-yl-, 1-(2-((4-((4-(aminocarbonyl)-1-piperidinyl)methyl)benzoyl)methylamino)ethyl)-4-piperidinyl Ester
15. Td-4208; Gsk-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate
16. Revefenacin [mi]
17. Revefenacin (usan/inn)
18. Revefenacin [usan:inn]
19. Revefenacin [usan]
20. Unii-g2ae2ve07o
21. Revefenacin;gsk1160724
22. Schembl356480
23. Chembl3833319
24. Gtpl10129
25. Revefenacin [orange Book]
26. Dtxsid701027775
27. Hms3886k17
28. Amy16789
29. Bcp15793
30. Ex-a1722
31. S5258
32. Td4208
33. Akos037649398
34. Ccg-270187
35. Cs-7743
36. Db11855
37. Sb17262
38. Bs-18189
39. Hy-15851
40. Revefenacin; Td-4208; Gsk-1160724
41. D10978
42. A903445
43. Q27278649
44. Revefenacin; Td 4208; Td4208; Gsk-1160724; Gsk1160724; Gsk 1160724
45. 1-(2-(4-((4-carbamoylpiperidin-1-yl)methyl)-n-methylbenzamido)ethyl)piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate
46. 1211931-83-7
47. Td-4208; Td 4208; Td4208; Gsk-1160724; Gsk1160724;1-(2-(4-((4-carbamoylpiperidin-1-yl)methyl)-n-methylbenzamido)ethyl)piperidin-4-yl N-((1,1'-biphenyl)-2-yl)carbamate
| Molecular Weight | 597.7 g/mol |
|---|---|
| Molecular Formula | C35H43N5O4 |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 11 |
| Exact Mass | 597.33150487 g/mol |
| Monoisotopic Mass | 597.33150487 g/mol |
| Topological Polar Surface Area | 108 Ų |
| Heavy Atom Count | 44 |
| Formal Charge | 0 |
| Complexity | 918 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Revefenacin is indicated as an inhalation solution for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD). COPD is a growing disease being the third leading cause of death in the US. This disease is characterized by not fully reversible airflow limitation.
FDA Label
Revefenacin has been reported to produce a sustained, long-acting bronchodilation with lower anti-muscarinic-related side effects. In clinical trials, revefenacin demonstrated to be of a long duration of action and low systemic exposure in patients with COPD. Also, it was reported that a dose of 88 mcg can produce a clinically effective bronchodilation measured by through forced expiratory volume in 1s and serial spirometric assessments. In placebo-controlled trials, revefenacin showed a decrease in the use of albuterol rescue inhalers and sustained increases in the peak expiratory flow rate that reached a steady state at a maximum in day 7. As well, there was a reported superior lung selectivity index when compared with other LABAs such as glycopyrronium and tiotropium which produced a decreased sialagogue effect.
R - Respiratory system
R03 - Drugs for obstructive airway diseases
R03B - Other drugs for obstructive airway diseases, inhalants
R03BB - Anticholinergics
R03BB08 - Revefenacin
Absorption
In pharmacokinetic studies, revefenacin was absorbed very rapidly and presented a linear increase in plasma exposure with Cmax, tmax and AUC that ranged between 0.02-0.15 ng/ml, 0.48-0.51 hours and 0.03-0.36 ng.h/ml, respectively. The bioaccumulation of revefenacin was very limited and the steady-state was achieved by day 7.
Route of Elimination
After reaching maximum concentration, revefenacin concentrations decline in a biphasic manner. This elimination kinetics is observed by a rapid declining plasma concentration followed by a slow apparent bi-exponential elimination. Renal elimination of revefenacin is limited and it presents a mean cumulative amount excreted in urine as the unchanged drug of < 0.2% of the administered dose. Following intravenous revefenacin administration, 54% of the dose is recovered in feces and 27% was recovered in urine which confirms a high hepatobiliary processing.
Volume of Distribution
After intravenous administration of revefenacin, the reported volume of distribution is 218 L which suggests an extensive distribution to the tissues.
Clearance
The renal clearance of revefenacin is negligible and thus, the clearance rate is not a major parameter for this drug.
Revefenacin presents a high metabolic liability producing a rapid metabolic turnover after being distributed from the lung. This metabolic process is done primarily via enzymatic hydrolysis via CYP2D6 to its major hydrolytic metabolite THRX-195518.
The apparent terminal half-life of a dose of 350 mcg of revefenacin was 22.3-70 hours.
Revefenacin is an inhaled bronchodilator muscarinic antagonist with a long-acting bronchodilation activity. It has been shown to present a high affinity and behaved as a competitive antagonist of the five muscarinic cholinergic receptors. Studies have indicated that revefenacin dissociates significantly slower from the muscarinic receptor M3 (hM3) when compared to the receptor M2 (hM2) which indicates a kinetic selectivity for this subtype. This competitive antagonism produces a suppressive action of the acetylcholine-evoked calcium mobilization and contractile responses in the airway tissue. Lastly, due to the duration of the bronchodilation, revefenacin is considered a long-acting muscarinic antagonist which allows it to be dosed once daily. This response is very important for the therapy of COPD as the main goal is the reduce the frequency and severity of exacerbations which are normally driven by the presence of elevated cholinergic bronchoconstrictor tone mediated by muscarinic receptors on parasympathetic ganglia and airway smooth muscle. Hence, the activity of revefenacin produces a potent and long-lasting protection against the bronchoconstrictor response to acetylcholine or methacholine.
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
73
PharmaCompass offers a list of Revefenacin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Revefenacin manufacturer or Revefenacin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Revefenacin manufacturer or Revefenacin supplier.
PharmaCompass also assists you with knowing the Revefenacin API Price utilized in the formulation of products. Revefenacin API Price is not always fixed or binding as the Revefenacin Price is obtained through a variety of data sources. The Revefenacin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate, including repackagers and relabelers. The FDA regulates TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate supplier is an individual or a company that provides TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate active pharmaceutical ingredient (API) or TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate finished formulations upon request. The TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate suppliers may include TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate API manufacturers, exporters, distributors and traders.
click here to find a list of TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate DMF (Drug Master File) is a document detailing the whole manufacturing process of TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate active pharmaceutical ingredient (API) in detail. Different forms of TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate DMFs exist exist since differing nations have different regulations, such as TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate DMF submitted to regulatory agencies in the US is known as a USDMF. TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate USDMF includes data on TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate suppliers with USDMF on PharmaCompass.
A TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate written confirmation (TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate WC) is an official document issued by a regulatory agency to a TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate manufacturer, verifying that the manufacturing facility of a TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate APIs or TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate finished pharmaceutical products to another nation, regulatory agencies frequently require a TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate WC (written confirmation) as part of the regulatory process.
click here to find a list of TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate suppliers with NDC on PharmaCompass.
TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate GMP manufacturer or TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate GMP API supplier for your needs.
A TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate CoA (Certificate of Analysis) is a formal document that attests to TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate's compliance with TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate specifications and serves as a tool for batch-level quality control.
TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate CoA mostly includes findings from lab analyses of a specific batch. For each TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate may be tested according to a variety of international standards, such as European Pharmacopoeia (TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate EP), TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (TD-4208; GSK-1160724;[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate USP).
