
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
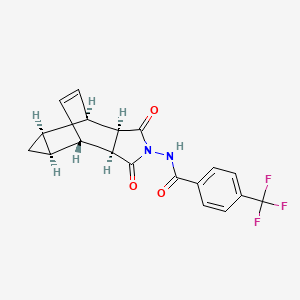

1. 4-trifluoromethyl-n-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop(f)isoindol-2(1h)-yl)-benzamide
2. Benzamide, N-((3ar,4r,4ar,5as,6s,6as)-3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop(f)isoindol-2(1h)-yl)-4-(trifluoromethyl)-, Hydrate (1:1), Rel-
3. N-((3ar,4r,4ar,5as,6s,6as)-1,3-dioxo-3,3a,4,4a,5,5a,6,6a-octahydro-4,6-ethenocyclopropa(f)isoindol-2(1h)-yl)-4-(trifluoromethyl)benzamide
4. Siga-246
5. St 246
6. St-246
7. Tecovirimat Monohydrate
8. Tpoxx
1. 869572-92-9
2. Siga-246
3. St 246
4. Tpoxx
5. St-246
6. F925rr824r
7. 816458-31-8
8. N-(3,5-dioxo-4-azatetracyclo[5.3.2.02,6.08,10]dodec-11-en-4-yl)-4-(trifluoromethyl)benzamide
9. N-[(1r,2r,6s,7s,8s,10r)-3,5-dioxo-4-azatetracyclo[5.3.2.02,6.08,10]dodec-11-en-4-yl]-4-(trifluoromethyl)benzamide
10. Benzamide, N-((3ar,4r,4ar,5as,6s,6as)-3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop(f)isoindol-2(1h)-yl)-4-(trifluoromethyl)-, Rel-
11. Tecovirimat [usan]
12. Tecovirimat [usan:inn]
13. Unii-f925rr824r
14. Siga 246
15. 4-trifluoromethyl-n-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop(f)isoindol-2(1h)-yl)-benzamide
16. Benzamide, N-[(3ar,4r,4ar,5as,6s,6as)-3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1h)-yl]-4-(trifluoromethyl)-, Rel-
17. N-((3ar,4r,4ar,5as,6s,6as)-1,3-dioxo-3,3a,4,4a,5,5a,6,6a-octahydro-4,6-ethenocyclopropa(f)isoindol-2(1h)-yl)-4-(trifluoromethyl)benzamide
18. Tecovirimat [mi]
19. Tecovirimat [inn]
20. Tecovirimat [who-dd]
21. Schembl404743
22. Arestvyr;iga-246;t-246
23. Chembl1257073
24. Schembl21670085
25. Tecovirimat [orange Book]
26. Dtxsid101026474
27. Ex-a4340
28. Bdbm50577060
29. S3380
30. St-246st-246
31. Zinc35323125
32. Akos030260536
33. Cs-3464
34. Db12020
35. Hy-14805
36. N-(dioxo[?]yl)-4-(trifluoromethyl)benzamide
37. 458t318
38. Q7692792
39. N-[(3ar,4r,4ar,5as,6s,6as)-1,3-dioxooctahydro-4,6-ethenocyclopropa[f]isoindol-2(1h)-yl]-4-(trifluoromethyl)benzamide
| Molecular Weight | 376.3 g/mol |
|---|---|
| Molecular Formula | C19H15F3N2O3 |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 1 |
| Exact Mass | 376.10347683 g/mol |
| Monoisotopic Mass | 376.10347683 g/mol |
| Topological Polar Surface Area | 66.5 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 705 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Tecovirimat is an inhibitor of the orthopoxvirus VP37 envelope wrapping protein and is indicated for the treatment of human smallpox disease in adults and pediatric patients weighing at least 13 kg. The efficacy of tecovirimat may be reduced in immunocompromised patients.
FDA Label
Tecovirimat SIGA is indicated for the treatment of the following viral infections in adults and children with body weight at least 13 kg:
- Smallpox
- Monkeypox
- Cowpox
Tecovirimat SIGA is also indicated to treat complications due to replication of vaccinia virus following vaccination against smallpox in adults and children with body weight at least 13 kg (see sections 4. 4 and 5. 1).
Tecovirimat SIGA should be used in accordance with official recommendations.
Tecovirimat prevents viral spread throughout the body. This drug inhibits its molecular target, a protein called p37, from interacting with intracellular transport components necessary for the production of enveloped virus, and therefore the spread of virus.
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
J05AX24
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AX - Other antivirals
J05AX24 - Tecovirimat
Absorption
Readily absorbed following oral administration, with mean times to maximum concentration from 3 to 4 h. A study was conducted to determine the safety, tolerability, and pharmacokinetics of ST-246 administered as a single daily oral dose. Steady state was reached by day 6 (within 3 to 5 half-lives).
Route of Elimination
Less than 0.02% of the drug is excreted unchanged in the kidney, with a majority of the drug being excreted in glucuronidated form. Approximately 23% of unchanged drug is found in the feces.
Volume of Distribution
Approximately 1,356 L. Following oral administration in mice, [14C]-tecovirimat was distributed to all tissues analyzed with the highest concentrations noted in liver and gallbladder, respiratory tract tissues (i.e., nasal turbinates), and bone marrow. Studies in dogs and NHPs suggest that tecovirimat crosses the blood-brain barrier.
Clearance
Mainly renal.
In vitro studies indicate that tecovirimat is not a substrate of major cytochrome P450 (CYP) enzymes, but it is a substrate of human recombinant UGTs (specifically of UGT1A1 and 1A4). Tecovirimat was found to be metabolized into 3 most abundant metabolites, M4, M5 and TFMBA, which do not have pharmacological activity.
Approximately 20h.
Tecovirimat inhibits the production of extracellular viral forms, which are responsible for the systemic spread of infection, inhibiting virus-induced cytopathic effects. Tecovirimat does not inhibit the formation of intracellular forms of the virus (IMV); however, by inhibiting envelopment, and therefore preventing the exit of viral particles from an infected cell, the smallpox infection is slowed to a point where the immune system can eliminate the virus. Tecovirimat has shown a high level of selectivity and specificity for orthopoxviruses. Tecovirimat targets the viral p37 protein, a highly conserved protein with no homologs outside of the Orthopoxvirus genus, inhibiting its function that is necessary for required for the viral envelopment of IMV (intracellular mature virus). Tecovirimat interferes with the cellular localization of p37 viral protein and prevents its association with cellular proteins involved in membrane trafficking.
Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
47
PharmaCompass offers a list of Tecovirimat API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Tecovirimat manufacturer or Tecovirimat supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Tecovirimat manufacturer or Tecovirimat supplier.
PharmaCompass also assists you with knowing the Tecovirimat API Price utilized in the formulation of products. Tecovirimat API Price is not always fixed or binding as the Tecovirimat Price is obtained through a variety of data sources. The Tecovirimat Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tecovirimat manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tecovirimat, including repackagers and relabelers. The FDA regulates Tecovirimat manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tecovirimat API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Tecovirimat manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Tecovirimat supplier is an individual or a company that provides Tecovirimat active pharmaceutical ingredient (API) or Tecovirimat finished formulations upon request. The Tecovirimat suppliers may include Tecovirimat API manufacturers, exporters, distributors and traders.
click here to find a list of Tecovirimat suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Tecovirimat DMF (Drug Master File) is a document detailing the whole manufacturing process of Tecovirimat active pharmaceutical ingredient (API) in detail. Different forms of Tecovirimat DMFs exist exist since differing nations have different regulations, such as Tecovirimat USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Tecovirimat DMF submitted to regulatory agencies in the US is known as a USDMF. Tecovirimat USDMF includes data on Tecovirimat's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Tecovirimat USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Tecovirimat suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Tecovirimat as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Tecovirimat API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Tecovirimat as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Tecovirimat and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Tecovirimat NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Tecovirimat suppliers with NDC on PharmaCompass.
Tecovirimat Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tecovirimat GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tecovirimat GMP manufacturer or Tecovirimat GMP API supplier for your needs.
A Tecovirimat CoA (Certificate of Analysis) is a formal document that attests to Tecovirimat's compliance with Tecovirimat specifications and serves as a tool for batch-level quality control.
Tecovirimat CoA mostly includes findings from lab analyses of a specific batch. For each Tecovirimat CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tecovirimat may be tested according to a variety of international standards, such as European Pharmacopoeia (Tecovirimat EP), Tecovirimat JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tecovirimat USP).
