
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
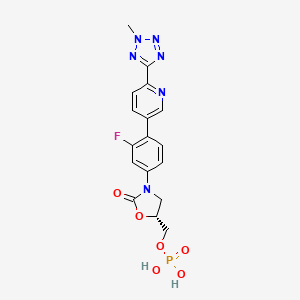

1. Da 7218
2. Da-7218
3. Da7218
4. Sivextro
5. Torezolid Phosphate
6. Tr 701
7. Tr-701
1. 856867-55-5
2. Torezolid Phosphate
3. Tr-701fa
4. Tr-701 Fa
5. Tedizolid (phosphate)
6. Tedizolid Phosphate [usan]
7. (r)-(3-(3-fluoro-4-(6-(2-methyl-2h-tetrazol-5-yl)pyridin-3-yl)phenyl)-2-oxooxazolidin-5-yl)methyl Dihydrogen Phosphate
8. Chebi:83326
9. Tr-701-fa
10. O7drj6r4dw
11. Tr-701
12. 856867-55-5 (phosphate)
13. [(5r)-3-[3-fluoro-4-[6-(2-methyltetrazol-5-yl)pyridin-3-yl]phenyl]-2-oxo-1,3-oxazolidin-5-yl]methyl Dihydrogen Phosphate
14. Tedizolidphosphate
15. [(5r)-3-{3-fluoro-4-[6-(2-methyl-2h-tetrazol-5-yl)pyridin-3-yl]phenyl}-2-oxo-1,3-oxazolidin-5-yl]methyl Dihydrogen Phosphate
16. Unii-o7drj6r4dw
17. Tedizolid-phosphate
18. Sivextro (tn)
19. Da-7218 Free Acid
20. Schembl1557561
21. Chembl2105669
22. Tedizolid Phosphate [mi]
23. Tedizolid Phosphate (jan/usan)
24. Amy9256
25. Dtxsid30234977
26. Tedizolid Phosphate [jan]
27. Tedizolid Phosphate [vandf]
28. Bcp10960
29. Ex-a5792
30. Tr-701 Free Acid Phosphate
31. Bdbm50017198
32. Da7218
33. Hy-14855b
34. Mfcd28098176
35. S4641
36. Tedizolid Phosphate [who-dd]
37. Zinc43100953
38. Akos027250820
39. Ccg-269233
40. Cs-5004
41. Db09042
42. Ncgc00482851-02
43. Tedizolid Phosphate [orange Book]
44. As-57141
45. D09686
46. Tr701-fa; Tr-701-fa; Tr 701-fa
47. A863474
48. Q21011227
49. ((5r)-3-(3-fluoro-4-(6-(2-methyl-2h-tetrazol-5-yl)pyridin-3-yl)phenyl)-2-oxooxazolidin-5- Yl)methyl Hydrogen Phosphate
50. ((5r)-3-(3-fluoro-4-(6-(2-methyl-2h-tetrazol-5-yl)pyridin-3-yl)phenyl)-2-oxooxazolidin-5-yl)methyl Hydrogen Phosphate
51. 2-oxazolidinone, 3-(3-fluoro-4-(6-(2-methyl-2h-tetrazol-5-yl)-3-pyridinyl)phenyl)-5- ((phosphonooxy)methyl)-, (5r)-
52. 2-oxazolidinone, 3-(3-fluoro-4-(6-(2-methyl-2h-tetrazol-5-yl)-3-pyridinyl)phenyl)-5-((phosphonooxy)methyl)-, (5r)-
| Molecular Weight | 450.3 g/mol |
|---|---|
| Molecular Formula | C17H16FN6O6P |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 6 |
| Exact Mass | 450.08529741 g/mol |
| Monoisotopic Mass | 450.08529741 g/mol |
| Topological Polar Surface Area | 153 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 702 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Tedizolid is indicated for the treatment of acute bacterial infections of the skin and skin structure (ABSSSI). To prevent drug resistance, tedizolid should only be used for infections that are caused by susceptible bacteria.
FDA Label
Sivextro is indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI) in adults and adolescents 12 years of age and older.
Treatment of acute bacterial skin and skin structure infections
Tedizolid is an oxazolidinone antibiotic that works by inhibiting protein synthesis by bacterial ribosomes. However, oxazolidinone antibiotics can also bind to human mitochondrial, but not cytoplasmic, ribosomes. Mitochondrial protein synthesis inhibition is associated with adverse patient effects such as neurological, hematological, and gastrointestinal toxicity, although tedizolid is tolerated better than the related [linezolid]. Alternative therapies should be considered when treating neutropenic patients with ABSSSI. _Clostridium difficile_-associated diarrhea has been reported in patients treated with tedizolid.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01XX11
Absorption
Tedizolid reaches peak plasma concentrations within three hours for oral administration and within one hour following intravenous administration; the absolute oral bioavailability is approximately 91%. Food has no effect on absorption. When given once daily, either orally or intravenously, tedizolid reaches steady-state concentrations in approximately three days. The Cmax for tedizolid after a single dose/at steady-state is 2.0 0.7/2.2 0.6 mcg/mL for oral administration, and 2.3 0.6/3.0 0.7 mcg/mL for intravenous administration, respectively. Similarly, the Tmax has a median (range) of 2.5 (1.0 - 8.0)/3.5 (1.0 - 6.0) hrs for the oral route and 1.1 (0.9 - 1.5)/1.2 (0.9 - 1.5) hrs when given intravenous. The AUC is 23.8 6.8/25.6 8.4 mcg\*hr/mL for oral and 26.6 5.2/29.2 6.2 mcg\*hr/mL for intravenous.
Route of Elimination
When given as a single oral dose, approximately 82% of tedizolid is excreted via the feces and 18% in urine. The majority is found as the inactive sulphate conjugate, with only 3% recovered unchanged. Over 85% of the elimination occurs within 96 hours.
Volume of Distribution
The volume of distribution for tedizolid following a single intravenous dose of 200 mg is between 67 and 80 L. In a study involving oral administration of 200 mg tedizolid to steady-state, the volume of distribution was 108 21 L, while a single 600 mg oral dose resulted in an apparent volume of distribution of 113.3 19.3 L. Tedizolid has been observed to penetrate the interstitial space of both adipose and skeletal muscle tissue and is also found in the epithelial lining fluid as well as in alveolar macrophages.
Clearance
Tedizolid has an apparent oral clearance of 6.9 1.7 L/hr for a single dose and 8.4 2.1 L/hr at steady-state. The systemic clearance is 6.4 1.2 L/hr for a single dose and 5.9 1.4 L/hr at steady-state.
Tedizolid is administered as a phosphate prodrug that is converted to tedizolid (the circulating active moiety). Prior to excretion, the majority of tedizolid is converted to an inactive sulphate conjugate in the liver, though this is unlikely to involve the action of cytochrome P450-family enzymes.
Tedizolid has a half-life of approximately 12 hours.
Despite renewed efforts to combat the spread of antimicrobial resistance, multidrug-resistant organisms, including gram-positive bacteria such as methicillin-resistant _Staphylococcus aureus_, remain a threat. Oxazolidinones represent a relatively new class of antibacterials inhibiting protein synthesis that is generally capable of overcoming resistance to other bacterial protein synthesis inhibitors. Protein synthesis involves the action of ribosomes, multi-subunit complexes composed of both protein and ribosomal RNA (rRNA) substituents. Translocation along the length of a messenger RNA and concomitant protein synthesis involves the action of the A, P, and E sites of the peptidyltransferase centre (PTC), which accepts charged aminoacyl-tRNAs and catalyzes the formation of peptide bonds between them. The bacterial 70S ribosome comprises a small (30S) and a large (50S) subunit. Early studies into the mechanism of action of oxazolidinone antibiotics suggested that they inhibit a step in the initiation of protein synthesis. However, this mechanism was inconsistent with mapped resistance mutations, and later studies involving cross-linking and direct structural determination of the binding site revealed that oxazolidinones, including both [linezolid] and tedizolid, bind in the A site of the PTC by interacting with the 23S rRNA component. The structural studies also revealed that oxazolidinone binding alters the conformation of a conserved nucleotide in the 23S rRNA (U2585 in _Escherichia coli_), which renders the PTC non-productive for peptide bond formation. Hence, tedizolid exerts its effect through inhibiting bacterial protein synthesis.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
91
PharmaCompass offers a list of Tedizolid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Tedizolid manufacturer or Tedizolid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Tedizolid manufacturer or Tedizolid supplier.
PharmaCompass also assists you with knowing the Tedizolid API Price utilized in the formulation of products. Tedizolid API Price is not always fixed or binding as the Tedizolid Price is obtained through a variety of data sources. The Tedizolid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tedizolid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tedizolid, including repackagers and relabelers. The FDA regulates Tedizolid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tedizolid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Tedizolid manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Tedizolid supplier is an individual or a company that provides Tedizolid active pharmaceutical ingredient (API) or Tedizolid finished formulations upon request. The Tedizolid suppliers may include Tedizolid API manufacturers, exporters, distributors and traders.
click here to find a list of Tedizolid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Tedizolid DMF (Drug Master File) is a document detailing the whole manufacturing process of Tedizolid active pharmaceutical ingredient (API) in detail. Different forms of Tedizolid DMFs exist exist since differing nations have different regulations, such as Tedizolid USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Tedizolid DMF submitted to regulatory agencies in the US is known as a USDMF. Tedizolid USDMF includes data on Tedizolid's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Tedizolid USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Tedizolid suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Tedizolid as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Tedizolid API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Tedizolid as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Tedizolid and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Tedizolid NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Tedizolid suppliers with NDC on PharmaCompass.
Tedizolid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tedizolid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tedizolid GMP manufacturer or Tedizolid GMP API supplier for your needs.
A Tedizolid CoA (Certificate of Analysis) is a formal document that attests to Tedizolid's compliance with Tedizolid specifications and serves as a tool for batch-level quality control.
Tedizolid CoA mostly includes findings from lab analyses of a specific batch. For each Tedizolid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tedizolid may be tested according to a variety of international standards, such as European Pharmacopoeia (Tedizolid EP), Tedizolid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tedizolid USP).
