

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
VMF
0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
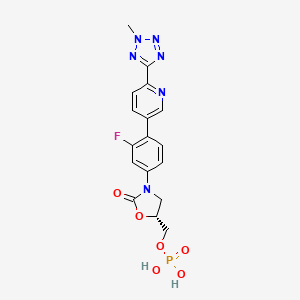

1. Da 7218
2. Da-7218
3. Da7218
4. Sivextro
5. Torezolid Phosphate
6. Tr 701
7. Tr-701
1. 856867-55-5
2. Torezolid Phosphate
3. Tr-701fa
4. Tr-701 Fa
5. Tedizolid (phosphate)
6. Tedizolid Phosphate [usan]
7. (r)-(3-(3-fluoro-4-(6-(2-methyl-2h-tetrazol-5-yl)pyridin-3-yl)phenyl)-2-oxooxazolidin-5-yl)methyl Dihydrogen Phosphate
8. Chebi:83326
9. Tr-701-fa
10. O7drj6r4dw
11. Tr-701
12. 856867-55-5 (phosphate)
13. [(5r)-3-[3-fluoro-4-[6-(2-methyltetrazol-5-yl)pyridin-3-yl]phenyl]-2-oxo-1,3-oxazolidin-5-yl]methyl Dihydrogen Phosphate
14. Tedizolidphosphate
15. [(5r)-3-{3-fluoro-4-[6-(2-methyl-2h-tetrazol-5-yl)pyridin-3-yl]phenyl}-2-oxo-1,3-oxazolidin-5-yl]methyl Dihydrogen Phosphate
16. Unii-o7drj6r4dw
17. Tedizolid-phosphate
18. Sivextro (tn)
19. Da-7218 Free Acid
20. Schembl1557561
21. Chembl2105669
22. Tedizolid Phosphate [mi]
23. Tedizolid Phosphate (jan/usan)
24. Amy9256
25. Dtxsid30234977
26. Tedizolid Phosphate [jan]
27. Tedizolid Phosphate [vandf]
28. Bcp10960
29. Ex-a5792
30. Tr-701 Free Acid Phosphate
31. Bdbm50017198
32. Da7218
33. Hy-14855b
34. Mfcd28098176
35. S4641
36. Tedizolid Phosphate [who-dd]
37. Zinc43100953
38. Akos027250820
39. Ccg-269233
40. Cs-5004
41. Db09042
42. Ncgc00482851-02
43. Tedizolid Phosphate [orange Book]
44. As-57141
45. D09686
46. Tr701-fa; Tr-701-fa; Tr 701-fa
47. A863474
48. Q21011227
49. ((5r)-3-(3-fluoro-4-(6-(2-methyl-2h-tetrazol-5-yl)pyridin-3-yl)phenyl)-2-oxooxazolidin-5- Yl)methyl Hydrogen Phosphate
50. ((5r)-3-(3-fluoro-4-(6-(2-methyl-2h-tetrazol-5-yl)pyridin-3-yl)phenyl)-2-oxooxazolidin-5-yl)methyl Hydrogen Phosphate
51. 2-oxazolidinone, 3-(3-fluoro-4-(6-(2-methyl-2h-tetrazol-5-yl)-3-pyridinyl)phenyl)-5- ((phosphonooxy)methyl)-, (5r)-
52. 2-oxazolidinone, 3-(3-fluoro-4-(6-(2-methyl-2h-tetrazol-5-yl)-3-pyridinyl)phenyl)-5-((phosphonooxy)methyl)-, (5r)-
| Molecular Weight | 450.3 g/mol |
|---|---|
| Molecular Formula | C17H16FN6O6P |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 6 |
| Exact Mass | 450.08529741 g/mol |
| Monoisotopic Mass | 450.08529741 g/mol |
| Topological Polar Surface Area | 153 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 702 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Tedizolid is indicated for the treatment of acute bacterial infections of the skin and skin structure (ABSSSI). To prevent drug resistance, tedizolid should only be used for infections that are caused by susceptible bacteria.
FDA Label
Sivextro is indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI) in adults and adolescents 12 years of age and older.
Treatment of acute bacterial skin and skin structure infections
Tedizolid is an oxazolidinone antibiotic that works by inhibiting protein synthesis by bacterial ribosomes. However, oxazolidinone antibiotics can also bind to human mitochondrial, but not cytoplasmic, ribosomes. Mitochondrial protein synthesis inhibition is associated with adverse patient effects such as neurological, hematological, and gastrointestinal toxicity, although tedizolid is tolerated better than the related [linezolid]. Alternative therapies should be considered when treating neutropenic patients with ABSSSI. _Clostridium difficile_-associated diarrhea has been reported in patients treated with tedizolid.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01XX11
Absorption
Tedizolid reaches peak plasma concentrations within three hours for oral administration and within one hour following intravenous administration; the absolute oral bioavailability is approximately 91%. Food has no effect on absorption. When given once daily, either orally or intravenously, tedizolid reaches steady-state concentrations in approximately three days. The Cmax for tedizolid after a single dose/at steady-state is 2.0 0.7/2.2 0.6 mcg/mL for oral administration, and 2.3 0.6/3.0 0.7 mcg/mL for intravenous administration, respectively. Similarly, the Tmax has a median (range) of 2.5 (1.0 - 8.0)/3.5 (1.0 - 6.0) hrs for the oral route and 1.1 (0.9 - 1.5)/1.2 (0.9 - 1.5) hrs when given intravenous. The AUC is 23.8 6.8/25.6 8.4 mcg\*hr/mL for oral and 26.6 5.2/29.2 6.2 mcg\*hr/mL for intravenous.
Route of Elimination
When given as a single oral dose, approximately 82% of tedizolid is excreted via the feces and 18% in urine. The majority is found as the inactive sulphate conjugate, with only 3% recovered unchanged. Over 85% of the elimination occurs within 96 hours.
Volume of Distribution
The volume of distribution for tedizolid following a single intravenous dose of 200 mg is between 67 and 80 L. In a study involving oral administration of 200 mg tedizolid to steady-state, the volume of distribution was 108 21 L, while a single 600 mg oral dose resulted in an apparent volume of distribution of 113.3 19.3 L. Tedizolid has been observed to penetrate the interstitial space of both adipose and skeletal muscle tissue and is also found in the epithelial lining fluid as well as in alveolar macrophages.
Clearance
Tedizolid has an apparent oral clearance of 6.9 1.7 L/hr for a single dose and 8.4 2.1 L/hr at steady-state. The systemic clearance is 6.4 1.2 L/hr for a single dose and 5.9 1.4 L/hr at steady-state.
Tedizolid is administered as a phosphate prodrug that is converted to tedizolid (the circulating active moiety). Prior to excretion, the majority of tedizolid is converted to an inactive sulphate conjugate in the liver, though this is unlikely to involve the action of cytochrome P450-family enzymes.
Tedizolid has a half-life of approximately 12 hours.
Despite renewed efforts to combat the spread of antimicrobial resistance, multidrug-resistant organisms, including gram-positive bacteria such as methicillin-resistant _Staphylococcus aureus_, remain a threat. Oxazolidinones represent a relatively new class of antibacterials inhibiting protein synthesis that is generally capable of overcoming resistance to other bacterial protein synthesis inhibitors. Protein synthesis involves the action of ribosomes, multi-subunit complexes composed of both protein and ribosomal RNA (rRNA) substituents. Translocation along the length of a messenger RNA and concomitant protein synthesis involves the action of the A, P, and E sites of the peptidyltransferase centre (PTC), which accepts charged aminoacyl-tRNAs and catalyzes the formation of peptide bonds between them. The bacterial 70S ribosome comprises a small (30S) and a large (50S) subunit. Early studies into the mechanism of action of oxazolidinone antibiotics suggested that they inhibit a step in the initiation of protein synthesis. However, this mechanism was inconsistent with mapped resistance mutations, and later studies involving cross-linking and direct structural determination of the binding site revealed that oxazolidinones, including both [linezolid] and tedizolid, bind in the A site of the PTC by interacting with the 23S rRNA component. The structural studies also revealed that oxazolidinone binding alters the conformation of a conserved nucleotide in the 23S rRNA (U2585 in _Escherichia coli_), which renders the PTC non-productive for peptide bond formation. Hence, tedizolid exerts its effect through inhibiting bacterial protein synthesis.
Registrant Name : Donga ST Co., Ltd.
Registration Date : 2015-04-17
Registration Number : Number 6401-9-ND
Manufacturer Name : AMRI Rensselaer
Manufacturer Address : 33 Riverside Avenue, Rensselaer, NY 12144, USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
NDC Package Code : 42765-022
Start Marketing Date : 2021-02-20
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
NDC Package Code : 65392-2912
Start Marketing Date : 2014-06-20
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (15kg/15kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 68225-066
Start Marketing Date : 2014-06-20
End Marketing Date : 2024-12-31
Dosage Form (Strength) : INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION (200mg/4mL)
Marketing Category : DRUG FOR FURTHER PROCESSING

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for the pharmaceutical and biotech industries. LGM is also a full service CDMO providing formulation, ...
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
About the Company : Established in 2004, Metrochem API is one of the fastest-growing APIs, pellets & intermediates manufacturers. It has 6 dedicated manufacturing facilities for its 3 core product ...
 Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
About the Company : Omgene Life Sciences Private Limited is an R&D-driven biopharmaceutical company specializing in biopharmaceuticals, peptides, semi-synthetic, and synthetic actives. As a vertically...
About the Company : Beijing Mesochem Technology Co. Ltd., which is located in the national economic and technological development area of Yizhuang, China, manufactures pharmaceutical chemicals, fine c...

About the Company : Guangzhou Tosun Pharmaceutical was founded in 1999, which mainly focuses on importation & exportation of Active Pharmaceutical Ingrediants, Chemical Raw Materials, Intermediate, Ex...

About the Company : Haoyuan Chemexpress Co., Ltd., located in Shanghai Zhangjiang Biomedical Base, is a high-tech company specializing in customized chemical synthesis and R&D of pharmaceutical interm...

About the Company : Hetero is a research based global pharmaceutical company focused on development, manufacturing and marketing of Active Pharmaceutical Ingredients (APIs), Intermediate Chemicals & F...

About the Company : Optimus is one of the fastest-growing pharmaceutical companies with 16 years of experience in providing the best quality of API, Intermediates & Finished formulations in Global mar...

About the Company : Shandong Hao Hong Biotechnology Co., Ltd. is located in Provincial Chemical Industrial Park of Liaocheng city, Shandong province, which is a beautiful water city. And recently anot...

About the Company : Viruj Pharmaceuticals, an India-based company with a global presence, aims to develop & market complex, technology-driven APIs. It offers contract API manufacturing for innovative ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Starizo (tedizolid phosphate) is an oxazolidinone-class antibacterial drug indicated in adult and pediatric patients 12 years of age and older for acute bacterial skin and skin structure infections.
Lead Product(s): Tedizolid
Therapeutic Area: Infections and Infectious Diseases Brand Name: Starizo
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Merck & Co
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Tedizolid
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Merck & Co
Deal Size : Not Applicable
Deal Type : Not Applicable
Sun Pharma Introduces a Novel Treatment, STARIZO in India for Bacterial Skin Infections
Details : Starizo (tedizolid phosphate) is an oxazolidinone-class antibacterial drug indicated in adult and pediatric patients 12 years of age and older for acute bacterial skin and skin structure infections.
Brand Name : Starizo
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Tedizolid phosphate is an oxazolidinone-class antibacterial drug indicated in adult patients for the treatment of acute bacterial skin and skin structure infections.
Lead Product(s): Tedizolid
Therapeutic Area: Infections and Infectious Diseases Brand Name: Tedizolid Phosphate-Generic
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 16, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Tedizolid
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Hetero Gets CDSCO Panel Nod to Study Tedizolid Phosphate for Bacterial Skin Infections
Details : Tedizolid phosphate is an oxazolidinone-class antibacterial drug indicated in adult patients for the treatment of acute bacterial skin and skin structure infections.
Brand Name : Tedizolid Phosphate-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 16, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Tedizolid phosphate is an oxazolidinone-class antibacterial drug indicated in adult patients for the treatment of acute bacterial skin and skin structure infections.
Lead Product(s): Tedizolid
Therapeutic Area: Infections and Infectious Diseases Brand Name: Tedizolid Phosphate-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 27, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Tedizolid
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Voluntary Announcement - Tedizolid Phosphate for Injection Obtains Approval
Details : Tedizolid phosphate is an oxazolidinone-class antibacterial drug indicated in adult patients for the treatment of acute bacterial skin and skin structure infections.
Brand Name : Tedizolid Phosphate-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 27, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the initial term of the agreement, Nabriva was solely responsible for marketing, sales, and distribution of SIVEXTRO (Tedizolid Phosphate) in the United States through December 31, 2023. The amendment extends the agreement to December 31, 2026.
Lead Product(s): Tedizolid
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sivextro
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Merck & Co
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Agreement May 05, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Tedizolid
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Merck & Co
Deal Size : Undisclosed
Deal Type : Agreement
Details : Under the initial term of the agreement, Nabriva was solely responsible for marketing, sales, and distribution of SIVEXTRO (Tedizolid Phosphate) in the United States through December 31, 2023. The amendment extends the agreement to December 31, 2026.
Brand Name : Sivextro
Molecule Type : Small molecule
Upfront Cash : Undisclosed
May 05, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the terms of the agreement, Nabriva will procure SIVEXTRO from Merck & Co. Inc., Kenilworth, N.J., USA and be responsible for marketing, sales, and distribution of SIVEXTRO in the U.S. through December 31, 2023, with renewable three-year extensions.
Lead Product(s): Tedizolid
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sivextro
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Merck & Co
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Agreement July 15, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Tedizolid
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Merck & Co
Deal Size : Undisclosed
Deal Type : Agreement
Nabriva Therapeutics Enters into Exclusive Agreement to Promote and Distribute SIVEXTRO® (tedizol...
Details : Under the terms of the agreement, Nabriva will procure SIVEXTRO from Merck & Co. Inc., Kenilworth, N.J., USA and be responsible for marketing, sales, and distribution of SIVEXTRO in the U.S. through December 31, 2023, with renewable three-year extensions...
Brand Name : Sivextro
Molecule Type : Small molecule
Upfront Cash : Undisclosed
July 15, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sivextro (tedizolid phosphate) is an oxazolidinone-class antibacterial drug indicated in adult and pediatric patients 12 years of age and older for acute bacterial skin and skin structure infections.
Lead Product(s): Tedizolid
Therapeutic Area: Infections and Infectious Diseases Brand Name: Sivextro
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 25, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Tedizolid
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Sivextro Approval Expanded to Include Pediatric Patients
Details : Sivextro (tedizolid phosphate) is an oxazolidinone-class antibacterial drug indicated in adult and pediatric patients 12 years of age and older for acute bacterial skin and skin structure infections.
Brand Name : Sivextro
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 25, 2020

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]5-Bromo-2-(2-methyl-2h-tetrazol-5-yl)pyridine
CAS Number : 380380-64-3
End Use API : Tedizolid
About The Company : Beijing Zhongshuo Pharmaceutical Technology Development Co., Ltd. (BJ Zhongshuo) was established in 2001. It came from a subsidiary company of China National Ph...

(R)-3-(4-Bromo-3-fluorophenyl)-5-(hydroxymethyl)ox...
CAS Number : 444335-16-4
End Use API : Tedizolid
About The Company : Beijing Zhongshuo Pharmaceutical Technology Development Co., Ltd. (BJ Zhongshuo) was established in 2001. It came from a subsidiary company of China National Ph...

Benzyl (4-bromo-3-fluorophenyl)carbamate
CAS Number : 510729-01-8
End Use API : Tedizolid
About The Company : Beijing Zhongshuo Pharmaceutical Technology Development Co., Ltd. (BJ Zhongshuo) was established in 2001. It came from a subsidiary company of China National Ph...

(4-(((Benzyloxy)carbonyl)amino)-2-fluorophenyl)bor...
CAS Number : 874290-59-2
End Use API : Tedizolid
About The Company : Beijing Zhongshuo Pharmaceutical Technology Development Co., Ltd. (BJ Zhongshuo) was established in 2001. It came from a subsidiary company of China National Ph...

(5R)-3-[3-fluoro-4-[6-(2-methyltetrazol-5-yl)pyrid...
CAS Number : 856866-72-3
End Use API : Tedizolid
About The Company : Beijing Zhongshuo Pharmaceutical Technology Development Co., Ltd. (BJ Zhongshuo) was established in 2001. It came from a subsidiary company of China National Ph...

2-(2-methyltetrazole-5 -yl )-5 -bromopyridine
CAS Number : 380380-64-3
End Use API : Tedizolid
About The Company : Established in 2011 and situated in Hangzhou, Zhejiang, China, Hangzhou Longshine Bio-Tech CO., Ltd is dedicated to providing services for pharmaceutical and ch...

(5R)-3 -(4-Bromo-3 -Fluorophenyl)-5 -Hydroxymethyl...
CAS Number : 444335-16-4
End Use API : Tedizolid
About The Company : Established in 2011 and situated in Hangzhou, Zhejiang, China, Hangzhou Longshine Bio-Tech CO., Ltd is dedicated to providing services for pharmaceutical and ch...

CAS Number : 97483-77-7
End Use API : Tedizolid
About The Company : Keminntek Laboratories is a Hyderabad (India) based Contract Research Organization in Pharmaceutical sector in specific Pharmaceutical Intermediates, Speciality...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : SIVEXTRO
Dosage Form : TABLET;ORAL
Dosage Strength : 200MG
Packaging :
Approval Date : 2014-06-20
Application Number : 205435
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : SIVEXTRO
Dosage Form : POWDER;INTRAVENOUS
Dosage Strength : 200MG/VIAL
Packaging :
Approval Date : 2014-06-20
Application Number : 205436
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Sivextro
Dosage Form : POWDER FOR CONCENTRATE FOR
Dosage Strength : 200 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Sivextro
Dosage Form : FILM COATED PILL
Dosage Strength : 200 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Sivextro
Dosage Form : Powder to concentrate to the infusion fluid, resolution
Dosage Strength : 200 mg
Packaging : Hood glass
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Sivextro
Dosage Form : Antic-calc Tablet, Film Coated
Dosage Strength : 200 mg
Packaging : Blister, endose
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : SIVEXTRO POWDER FOR SOLUTION FOR INFUSION
Dosage Form : INF
Dosage Strength : 200mg
Packaging : 6X1mg
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : SIVEXTRO 200 mg TABLET
Dosage Form : FCT
Dosage Strength : 200mg
Packaging : 6X1mg
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : SIVEXTRO
Dosage Form : TABLET;ORAL
Dosage Strength : 200MG
Approval Date : 2014-06-20
Application Number : 205435
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : SIVEXTRO
Dosage Form : POWDER;INTRAVENOUS
Dosage Strength : 200MG/VIAL
Approval Date : 2014-06-20
Application Number : 205436
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
