
Synopsis
Synopsis
0
CEP/COS
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
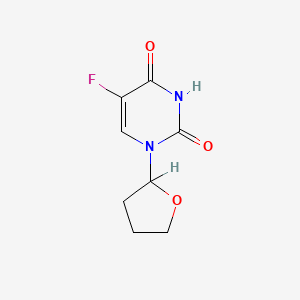

1. 1-(2-tetrahydrofuryl)-5-fluorouracil
2. 1-(tetrahydro-2-furanyl)-5-fluorouracil
3. 5-fluoro-1-(tetrahydro-2-furanyl)-2,4-pyrimidinedione
4. Florafur
5. Fluorofur
6. Ft 207
7. Ft-207
8. Ft207
9. Ftorafur
10. Futraful
11. N1-(2'-tetrahydrofuryl)-5-fluorouracil
12. Sunfural S
13. Uftoral
14. Utefos
1. Ftorafur
2. 17902-23-7
3. Futraful
4. Sinoflurol
5. Fental
6. Fluorofur
7. Citofur
8. Neberk
9. Coparogin
10. Furafluor
11. Furofutran
12. Nitobanil
13. Exonal
14. Fulfeel
15. Lifril
16. Lamar
17. Tefsiel C
18. Florafur
19. Riol
20. 5-fluoro-1-(tetrahydrofuran-2-yl)pyrimidine-2,4(1h,3h)-dione
21. Ft-207
22. Franroze
23. Furflucil
24. Sunfral
25. 1-(2-tetrahydrofuryl)-5-fluorouracil
26. Franrose
27. Phthorafur
28. Sunfural
29. Fulaid
30. 5-fluoro-1-(tetrahydro-2-furyl)uracil
31. Mjf-12264
32. 2,4(1h,3h)-pyrimidinedione, 5-fluoro-1-(tetrahydro-2-furanyl)-
33. Atillon
34. Fluorafur
35. Nsc-148958
36. 5-fluoro-1-(tetrahydro-2-furfuryl)uracil
37. Uracil, 5-fluoro-1-(tetrahydro-2-furyl)-
38. Ft 207
39. 5-fluoro-1-(tetrahydro-2-furanyl)-2,4-pyrimidinedione
40. N1-(2-tetrahydrofuryl)-5-fluorouracil
41. 1-(tetrahydrofuran-2-yl)-5-fluorouracil
42. 5-fluoro-1-(tetrahydrofuran-2-yl)uracil
43. Uracil, 1-(tetrahydrofuran-2-yl)-5-fluoro-
44. 5-fluoro-1-(oxolan-2-yl)pyrimidine-2,4-dione
45. Nsc 148958
46. 5-fluoro-1-(tetrahydro-3-furyl)uracil
47. 1-(tetrahydro-2-furanyl)-5-fluoro-2,4-pyrimidinedione
48. Mls000069497
49. Chebi:32188
50. 1-(2-tetrahydroformyl)-5-fluorouracil
51. 1548r74nsz
52. 5-fluoro-1-tetrahydrofuran-2-yl-pyrimidine-2,4-dione
53. Ncgc00159418-02
54. Ncgc00159418-05
55. Smr000059106
56. 2,4(1h,3h)pyrimidinedione, 5-fluoro-1-(tetrahydro-2-furanyl)-
57. Racemic Ftorafur
58. Dsstox_cid_1305
59. Dsstox_rid_76070
60. Dsstox_gsid_21305
61. Phthorafur [czech]
62. Ft-207 (nsc 148958)
63. Tegafurum
64. Tegafurum [inn-latin]
65. Cas-37076-68-9
66. Ccris 2762
67. Ft207
68. Einecs 241-846-2
69. Mfcd00012351
70. Brn 0525766
71. N1-(2'-tetrahydrofuryl)-5-fluorouracil
72. 1-(tetrahydro-2-furanyl)-5-fluorouracil
73. Unii-1548r74nsz
74. N(sub 1)-(2-tetrahydrofuryl)-5-fluorouracil
75. Tegafur [usan:inn:ban:jan]
76. N(sub 1)-(2'-furanidyl)-5-fluouracil [czech]
77. Atillon (tn)
78. Tegafur ,(s)
79. N(sub 1)-(2'-furanidyl)-5-fluouracil
80. Ts-1 (salt/mix)
81. Tegafur [usan]
82. Tegafur [inn]
83. Tegafur [jan]
84. Tegafur [mi]
85. Opera_id_1726
86. Tegafur [mart.]
87. Tegafur [who-dd]
88. Upcmld-dp063
89. Schembl4552
90. Tegafur [ema Epar]
91. 5-24-06-00285 (beilstein Handbook Reference)
92. 79107-97-4
93. Mls000759414
94. Mls001076521
95. Mls001424119
96. Chembl20883
97. Tegafur (jp17/usan/inn)
98. F-5-fu
99. Teysuno Component Tegafur
100. Upcmld-dp063:001
101. Gtpl10513
102. Dtxsid001009966
103. Hms1665i05
104. Hms2051b15
105. Hms2090k04
106. Hms2232e05
107. Hms3371h21
108. Hms3393b15
109. Hms3654p13
110. Hms3715d14
111. N1-(2'-furanidyl)-5-fluouracil
112. 5-fluoro-1-(tetrahydro-2-furanyl)-2,4(1h,3h)-pyrimidinedione
113. Bcp22714
114. Tegafur Component Of Teysuno
115. 5-fluoro-1-(tetrahydrofuran-2-yl)
116. N1-(2'-furanidyl)-5-fluorouracil
117. Tox21_111649
118. Tox21_301812
119. Bbl027795
120. Ccg-50110
121. Stk528044
122. Tegafur, >=98% (hplc), Powder
123. 5-fluoro-1-(2-tetrahydrofuryl)uracil
124. Akos000121279
125. Tox21_111649_1
126. Ac-2112
127. Ccg-100959
128. Cs-1128
129. Db09256
130. Nc00209
131. 1-(tetrahydro-2-furyl)-5-fluorouracil
132. 2,4(1h,3h)-pyrimidinedione, 5-fluoro-1-(tetrahydro-2-furanyl)-, Didehydroderiv.
133. 5-fluoro-1-(2-tetrahydrofuranyl)uracil
134. 5-fluoro-1-(tetrahydro-2-furyl)-uracil
135. N1 -(2-tetrahydrofuryl)-5-fluorouracil
136. Ncgc00159418-04
137. Ncgc00255222-01
138. 82294-77-7
139. As-13528
140. Hy-17400
141. Tegafur (ft-207; Nsc 148958)
142. Ft-0653732
143. Ft-0654170
144. Ft-0674829
145. Ft-0693965
146. D01244
147. Ab00572620-15
148. 902t237
149. A812417
150. Q413370
151. Sr-01000639511
152. Q-201784
153. Sr-01000639511-1
154. Sr-01000639511-4
155. 5-fluoro-1-tetrahydro-furan-2-yl-1h-pyrimidine-2,4-dione
156. 5-fluoro-1-tetrahydro-2-furanyl-2,4(1h,3h)-pyrimidinedione
157. 2, 4(1h,3h)-pyrimidinedione, 5-fluoro-1-(tetrahydro-2-furanyl)-, (r)-
| Molecular Weight | 200.17 g/mol |
|---|---|
| Molecular Formula | C8H9FN2O3 |
| XLogP3 | -0.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 200.05972032 g/mol |
| Monoisotopic Mass | 200.05972032 g/mol |
| Topological Polar Surface Area | 58.6 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 316 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for the treatment of cancer usually in combination with other biochemically modulating drugs. Indicated in adults for the treatment of advanced gastric cancer when given in combination with [DB00515]. Indicated for the first-line treatment of metastatic colorectal cancer with [DB03419] and calcium folinate.
Tegafur is an antineoplastic agent that belongs in the class of pyrimidine analogues. It interferes with the 2'-deoxythymidylate (DTMP) synthesis in the pyrimidine pathway, resulting in inhibition of DNA synthesis. In a phase III trial investigating the clinical efficacy of S-1 (tegafur/gimeracil/oteracil) in patients with advanced or recurrent gastric cancer, treatment resulted in a high response rate and was associated with a longer overall survival and longer progression-free survival rate when used in combination with cisplatin. In a meta analysis, triple combination therapy consisting of tegafur, gimeracil and oteracil showed longer survival times and well tolerance in patients with advanced gastric cancer. Tegafur and its active metabolites are potent myleosuppressive agents.
Antimetabolites, Antineoplastic
Antimetabolites that are useful in cancer chemotherapy. (See all compounds classified as Antimetabolites, Antineoplastic.)
L01BC53
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01B - Antimetabolites
L01BC - Pyrimidine analogues
L01BC03 - Tegafur
Absorption
Tegafur displays a dose-proportional pharmacokinetic properties. Tegafur is rapidly and well absorbed into the systemic circulation, reaching the peak plasma concentration within 1 to 2 hours of administration.
Route of Elimination
Following oral administration, about less 20% of total tegafur is excreted unchanged in the urine.
Volume of Distribution
The volume of distribution based on apparent volume of distribution and urinary excretion data of tegafur is 16 L/m^2.
Clearance
No pharmacokinetic data available.
Hepatic CYP2A6 is the predominant enzyme that mediates 5-hydroxylation of tegafur to generate 5'-hydroxytegafur. This metabolite is unstable and undergoes spontaneous degradation to form 5-FU, which is an active antineoplastic agent that exerts a pharmacological action on tumours. 5-FU is rapidly metabolised by the liver enzyme dihydropyrimidine dehydrogenase (DPD).
Tegafur has known human metabolites that include 5-Fluorouracil.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The elimination half life of tegafur is approximately 11 hours.
The transformation of 2'-deoxyurindylate (dUMP) to 2'-deoxythymidylate (dTMP) is essential in driving the synthesis of DNA and purines in cells. Thymidylate synthase catalyzes the conversion of dUMP to dTMP, which is a precursor of thymidine triphosphate (TTP), one of the four deoxyribonucleotides required for DNA synthesis. After administration into the body, tegafur is converted into the active antineoplastic metabolite, fluorouracil (5-FU). In tumour cells, 5-FU undergoes phosphorylation to form the active anabolites, including 5-fluorodeoxyuridine monophosphate (FdUMP). FdUMP and reduced folate are bound to thymidylate synthase leading to formation of a ternary complex which inhibits DNA synthesis. In addition, 5-fluorouridine-triphosphate (FUTP) is incorporated into RNA causing disruption of RNA functions.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

ABOUT THIS PAGE
59
PharmaCompass offers a list of Tegafur API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Tegafur manufacturer or Tegafur supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Tegafur manufacturer or Tegafur supplier.
PharmaCompass also assists you with knowing the Tegafur API Price utilized in the formulation of products. Tegafur API Price is not always fixed or binding as the Tegafur Price is obtained through a variety of data sources. The Tegafur Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tegafur manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tegafur, including repackagers and relabelers. The FDA regulates Tegafur manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tegafur API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Tegafur manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Tegafur supplier is an individual or a company that provides Tegafur active pharmaceutical ingredient (API) or Tegafur finished formulations upon request. The Tegafur suppliers may include Tegafur API manufacturers, exporters, distributors and traders.
click here to find a list of Tegafur suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Tegafur DMF (Drug Master File) is a document detailing the whole manufacturing process of Tegafur active pharmaceutical ingredient (API) in detail. Different forms of Tegafur DMFs exist exist since differing nations have different regulations, such as Tegafur USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Tegafur DMF submitted to regulatory agencies in the US is known as a USDMF. Tegafur USDMF includes data on Tegafur's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Tegafur USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Tegafur suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Tegafur Drug Master File in Japan (Tegafur JDMF) empowers Tegafur API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Tegafur JDMF during the approval evaluation for pharmaceutical products. At the time of Tegafur JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Tegafur suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Tegafur Drug Master File in Korea (Tegafur KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Tegafur. The MFDS reviews the Tegafur KDMF as part of the drug registration process and uses the information provided in the Tegafur KDMF to evaluate the safety and efficacy of the drug.
After submitting a Tegafur KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Tegafur API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Tegafur suppliers with KDMF on PharmaCompass.
A Tegafur written confirmation (Tegafur WC) is an official document issued by a regulatory agency to a Tegafur manufacturer, verifying that the manufacturing facility of a Tegafur active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Tegafur APIs or Tegafur finished pharmaceutical products to another nation, regulatory agencies frequently require a Tegafur WC (written confirmation) as part of the regulatory process.
click here to find a list of Tegafur suppliers with Written Confirmation (WC) on PharmaCompass.
Tegafur Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tegafur GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tegafur GMP manufacturer or Tegafur GMP API supplier for your needs.
A Tegafur CoA (Certificate of Analysis) is a formal document that attests to Tegafur's compliance with Tegafur specifications and serves as a tool for batch-level quality control.
Tegafur CoA mostly includes findings from lab analyses of a specific batch. For each Tegafur CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tegafur may be tested according to a variety of international standards, such as European Pharmacopoeia (Tegafur EP), Tegafur JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tegafur USP).
