


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
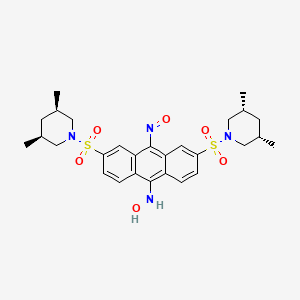

1. Tegatrabetan
2. 1227637-23-1
3. Bc2059
4. Tegavivint [inn]
5. Bc-2059
6. 18ap231hup
7. Chembl3601411
8. N-[3,6-bis[[(3s,5r)-3,5-dimethylpiperidin-1-yl]sulfonyl]-10-nitrosoanthracen-9-yl]hydroxylamine
9. 9,10-anthracenedione, 2,7-bis(((3r,5s)-3,5-dimethyl-1-piperidinyl)sulfonyl)-, 9,10-dioxime, Rel-
10. Tegavivint [who-dd]
11. Unii-18ap231hup
12. Schembl14947676
13. Bdbm50108103
14. Nsc785527
15. Nsc-785527
16. Bs-14778
17. Hy-109103
18. Cs-0039507
19. A14381
20. D71173
21. 2,7-bis(((3r,5s)-3,5-dimethylpiperidin-1-yl)sulfonyl)anthracene-9,10-dione Dioxime
22. 2,7-bis[((3r,5s)-3,5-dimethylpiperidine-1-yl)sulfonyl]anthracene-9,10-dione Dioxime
23. Rel-2,7-bis(((3r,5s)-3,5-dimethylpiperidin-1-yl)sulfonyl)anthracene-9,10-dione Dioxime
| Molecular Weight | 588.7 g/mol |
|---|---|
| Molecular Formula | C28H36N4O6S2 |
| XLogP3 | 5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 5 |
| Exact Mass | 588.20762723 g/mol |
| Monoisotopic Mass | 588.20762723 g/mol |
| Topological Polar Surface Area | 153 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 1020 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


