
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
VMF
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
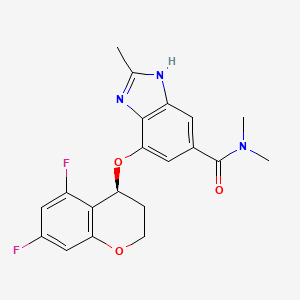

1. (s)-4-((5,7-difluorochroman-4-yl)oxy)-n,n,2-trimethyl-1h-benzo(d)imidazole-6-carboxamide
2. 1h-benzimidazole-5-carboxamide, 7-(((4s)-5,7-difluoro-3,4-dihydro-2h-1-benzopyran-4-yl)oxy)-n,n,2-trimethyl-
3. 7-(((4s)-5,7-difluoro-3,4-dihydro-2h-chromen-4-yl)oxy)-n,n,2-trimethyl-1h-benzimidazole-5-carboxamide
1. 942195-55-3
2. Tegoprazan [inn]
3. W017g7if4s
4. Lxi-15028
5. (s)-4-((5,7-difluorochroman-4-yl)oxy)-n,n,2-trimethyl-1h-benzo[d]imidazole-6-carboxamide
6. 1h-benzimidazole-5-carboxamide, 7-(((4s)-5,7-difluoro-3,4-dihydro-2h-1-benzopyran-4-yl)oxy)-n,n,2-trimethyl-
7. Unii-w017g7if4s
8. Emixustat Hcl
9. (s)-4-((5,7-difluorochroman-4-yl)oxy)-n,n,2-trimethyl-1h-benzo(d)imidazole-6-carboxamide
10. 7-[[(4s)-5,7-difluoro-3,4-dihydro-2h-chromen-4-yl]oxy]-n,n,2-trimethyl-3h-benzimidazole-5-carboxamide
11. K-cab
12. Tegoprazan [who-dd]
13. Schembl2687723
14. Chembl4297583
15. Schembl19236298
16. Gtpl12008
17. (s)-7-((5,7-difluorochroman-4-yl)oxy)-n,n,2-trimethyl-1h-benzo(d)imidazole-5-carboxamide
18. Ex-a4304
19. Cj12420
20. Ac-36576
21. As-84160
22. Cj-12420
23. Hy-17623
24. Cs-0014702
25. E83739
26. Cj-12420; In-a001; Lxi-15028
27. Q27292116
28. (-)-4-[((4s)-5,7-difluoro-3,4-dihydro-2h-chromen-4-yl)oxy]-n,n,2-trimethyl-1h-benzimidazole-6-carboxamide
29. 7-(((4s)-5,7-difluoro-3,4-dihydro-2h-chromen-4-yl)oxy)-n,n,2-trimethyl-1h-benzimidazole-5-carboxamide
30. 7-[[(4s)-5,7-difluoro-3,4-dihydro-2h-1-benzopyran-4-yl]oxy]-n,n,2-trimethyl-1h-benzimidazole-5-carboxamide
31. 8bn
| Molecular Weight | 387.4 g/mol |
|---|---|
| Molecular Formula | C20H19F2N3O3 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 387.13944780 g/mol |
| Monoisotopic Mass | 387.13944780 g/mol |
| Topological Polar Surface Area | 67.4 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 581 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
A - Alimentary tract and metabolism
A02 - Drugs for acid related disorders
A02B - Drugs for peptic ulcer and gastro-oesophageal reflux disease (gord)
A02BC - Proton pump inhibitors
A02BC09 - Tegoprazan
Tegoprazan works as a potassium-competitive acid blocker that is potent and highly selective. Its mechanism of action is different from that of the proton-pump inhibitors as this drug does not require conversion into an active form and can directly inhibit H+/K+ATPase in a reversible and K+competitive way. This is because it is an acid-resistant weak base with the ability to remain in the highly acidic canaliculi of gastric parietal cells.
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38991
Submission : 2023-12-30
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39217
Submission : 2023-11-29
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39156
Submission : 2023-11-18
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registrant Name : Dasan Pharmaceutical Co., Ltd.
Registration Date : 2024-01-15
Registration Number : 2243-13-ND
Manufacturer Name : SPC Co., Ltd. @ Dasan Pharma...
Manufacturer Address : 386 Pyeongtaek Port Road, Poseung-eup, Pyeongtaek-si, Gyeonggi-do @ 342 Deokamsan-ro,...

Registrant Name : HKINNOEN Co., Ltd.
Registration Date : 2021-04-16
Registration Number : 1452-6-ND
Manufacturer Name : HKINNOEN Co., Ltd.
Manufacturer Address : 20 Daesosandan-ro, Daeso-myeon, Eumseong-gun, Chungcheongbuk-do

Registrant Name : HKINNOEN Co., Ltd.
Registration Date : 2021-10-18
Registration Number : 1452-7-ND
Manufacturer Name : HKINNOEN Co., Ltd. @ST Pharm...
Manufacturer Address : 20 Daesosandan-ro, Daeso-myeon, Eumseong-gun, Chungcheongbuk-do @ 171 Haean-ro, Danwo...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Tegoprazan (CJ-12420) is a novel agent in development for the treatment of acid-related gastrointestinal diseases. Tegoprazan has already received marketing authorization in multiple territories, including South Korea and China.
Lead Product(s): Tegoprazan
Therapeutic Area: Gastroenterology Brand Name: CJ-12420
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Sebela Pharmaceuticals
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Acquisition October 19, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Tegoprazan
Therapeutic Area : Gastroenterology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Sebela Pharmaceuticals
Deal Size : Undisclosed
Deal Type : Acquisition
Sebela Pharmaceuticals® Acquires Exclusive Licensing Rights to Develop and Commercialize Tegopraz...
Details : Tegoprazan (CJ-12420) is a novel agent in development for the treatment of acid-related gastrointestinal diseases. Tegoprazan has already received marketing authorization in multiple territories, including South Korea and China.
Brand Name : CJ-12420
Molecule Type : Small molecule
Upfront Cash : Undisclosed
October 19, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under this partnership, HK inno.N Corporation will be responsible for the manufacture and supply of Tegoprazan (CJ-12420), while Dr. Reddy’s will be responsible for local clinical development, registration, marketing and sales in the licensed territories
Lead Product(s): Tegoprazan
Therapeutic Area: Gastroenterology Brand Name: CJ-12420
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Dr. Reddy\'s Laboratories
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership May 11, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Tegoprazan
Therapeutic Area : Gastroenterology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Dr. Reddy\'s Laboratories
Deal Size : Undisclosed
Deal Type : Partnership
Dr. Reddy’s Laboratories to Commercialise Novel Molecule Tegoprazan in India
Details : Under this partnership, HK inno.N Corporation will be responsible for the manufacture and supply of Tegoprazan (CJ-12420), while Dr. Reddy’s will be responsible for local clinical development, registration, marketing and sales in the licensed territori...
Brand Name : CJ-12420
Molecule Type : Small molecule
Upfront Cash : Undisclosed
May 11, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
In the study, cohorts with 50 and 100 milligrams of K-CAB tablets showed superior therapeutic effect and safety in treating gastric ulcers than the group administered with 30 milligrams of Lansoprazole.
Lead Product(s): Tegoprazan
Therapeutic Area: Gastroenterology Brand Name: K-CAB
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 24, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Tegoprazan
Therapeutic Area : Gastroenterology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Hk Inno.N Publishes P3 Trial Results of Drug on Gastric Ulcers
Details : In the study, cohorts with 50 and 100 milligrams of K-CAB tablets showed superior therapeutic effect and safety in treating gastric ulcers than the group administered with 30 milligrams of Lansoprazole.
Brand Name : K-CAB
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 24, 2020

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?