
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
Europe

0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
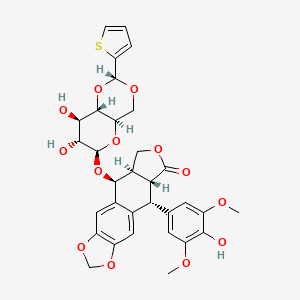

1. Demethyl Epipodophyllotoxin Thenylidine Glucoside
2. Nsc 122819
3. Nsc-122819
4. Nsc122819
5. Teniposide, (5a Alpha,9 Alpha(s*))-isomer
6. Vm 26
7. Vm-26
8. Vm26
9. Vumon
1. 29767-20-2
2. Vumon
3. Vehem
4. Vm-26
5. Nsc 122819
6. Vm26
7. Nsc-122819
8. Teniposidum
9. Teniposido
10. Teniposide (vumon)
11. Teniposidum [inn-latin]
12. Teniposido [inn-spanish]
13. Vm 26
14. 4'-demethylepipodophyllotoxin 9-(4,6-o-(r)-2-thenylidene-beta-d-glucopyranoside)
15. 957e6438qa
16. Hsdb 6546
17. 5-[[(6r)-7,8-dihydroxy-2-thiophen-2-yl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5h-[2]benzofuro[6,5-f][1,3]benzodioxol-8-one
18. Vee M-26
19. 4'-demethylepipodophyllotoxin 9-(4,6-o-2-thenylidene-beta-d-glucopyranoside)
20. Teniposide [usan]
21. Nsc122819
22. Ccris 2058
23. Einecs 249-831-2
24. 4'-dimethyl-9-(4,6-o-2-thenyid)-epipodophyllotoxin
25. Epidophyllotoxin
26. Teniposide [usan:inn:ban]
27. 4'-demethylepipodophyllotoxin-beta-d-thenylidine Glucoside
28. Mfcd00866516
29. 4'-demethyl-epipodophyllotoxin-beta-d-thenylidene-glucoside
30. Unii-957e6438qa
31. (5s,5ar,8ar,9r)-5-[[(2r,4ar,6r,7r,8r,8as)-7,8-dihydroxy-2-thiophen-2-yl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5h-[2]benzofuro[6,5-f][1,3]benzodioxol-8-one
32. Teniposide- Bio-x
33. Epipodophyllotoxin, 4'-demethyl-, 9-(4,6-o-2-thenylidene-beta-d-glucopyranoside)
34. Epipodophyllotoxin-beta-d-thenylidene-glucoside, 4'-demethyl-
35. Teniposide [mi]
36. Teniposide [inn]
37. Teniposide [hsdb]
38. Teniposide [iarc]
39. Teniposide [vandf]
40. Schembl3908
41. Teniposide [mart.]
42. 4'-demethyl 1-o-(4,6-o,o-(2-thenylidene)-beta-d-glucopyranosyl)epipodophyllotoxin
43. Teniposide [usp-rs]
44. Teniposide [who-dd]
45. (5r-(5alpha,5abeta,8aalpha,9beta(r*)))-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-9-((4-6-o-(2-thienylmethylene)-beta-d-glucopyranosyl)oxy)furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5ah)-one
46. Chembl452231
47. Gtpl6843
48. Dtxsid8023638
49. Teniposide [orange Book]
50. 4'-demethylepipodophyllotoxin 9-(4,6-o-(r)-2-thenylidene-.beta.-d-glucopyranoside)
51. Teniposide [usp Monograph]
52. Zinc4099009
53. Bdbm50248198
54. Nsc758255
55. S1787
56. Akos015896073
57. Ccg-270335
58. Cs-1366
59. Db00444
60. Ks-5147
61. Nsc-758255
62. Bt164452
63. Furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5ah)-one, 5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-9-((4,6-o-((r)-2-thienylmethylene)-beta-d-glucopyranosyl)oxy)-, (5r,5ar,8ar,9s)-
64. Furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5ah)-one, 5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-9-((4,6-o-(2-thienylmethylene)-beta-d-glucopyranosyl)-oxy)-, (5r-(5alpha,5abeta,8aalpha,9beta(r*)))-
65. Hy-13761
66. Ab01274729-01
67. Ab01274729_02
68. 767t202
69. Q417555
70. (5r,5ar,8ar,9s)-9-(((2r,4ar,6r,7r,8r,8as)-7,8-dihydroxy-2-(thiophen-2-yl)hexahydropyrano[3,2-d][1,3]dioxin-6-yl)oxy)-5-(4-hydroxy-3,5-dimethoxyphenyl)-5,5a,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(8h)-one
71. (5s,5ar,8ar,9r)-5-[[(2r,4ar,6r,7r,8r,8as)-7,8-dihydroxy-2-(2-thienyl)-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxy-phenyl)-5a,6,8a,9-tetrahydro-5h-isobenzofuro[6,5-f][1,3]benzodioxol-8-one
72. Furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5ah)-one, 5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-9-((4,6-o-(2-thienylmethylene)-.beta.-d-glucopyranosyl)-oxy)-, (5r-(5.alpha.,5a.beta.,8a.alpha.,9.beta.(r*)))
73. Furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5ah)-one, 5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-9-[[4,6-o-[(r)-2-thienylmethylene]-.beta.-d-glucopyranosyl]oxy]-, (5r,5ar,8ar,9s)-
| Molecular Weight | 656.7 g/mol |
|---|---|
| Molecular Formula | C32H32O13S |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 6 |
| Exact Mass | 656.15636224 g/mol |
| Monoisotopic Mass | 656.15636224 g/mol |
| Topological Polar Surface Area | 189 Ų |
| Heavy Atom Count | 46 |
| Formal Charge | 0 |
| Complexity | 1090 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Vumon |
| PubMed Health | Teniposide (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | VUMON (teniposide injection) (also commonly known as VM-26), is supplied as a sterile nonpyrogenic solution in a nonaqueous medium intended for dilution with a suitable parenteral vehicle prior to intravenous infusion. VUMON is available in 50 mg (... |
| Active Ingredient | Teniposide |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/ml |
| Market Status | Prescription |
| Company | Hq Speclt Pharma |
| 2 of 2 | |
|---|---|
| Drug Name | Vumon |
| PubMed Health | Teniposide (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | VUMON (teniposide injection) (also commonly known as VM-26), is supplied as a sterile nonpyrogenic solution in a nonaqueous medium intended for dilution with a suitable parenteral vehicle prior to intravenous infusion. VUMON is available in 50 mg (... |
| Active Ingredient | Teniposide |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/ml |
| Market Status | Prescription |
| Company | Hq Speclt Pharma |
Antineoplastic Agents; Enzyme Inhibitors; Nucleic Acid Synthesis Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Teniposide is indicated, in combination with other approved anticancer agents, for induction therapy of refractory childhood acute lymphocytic (lymphoblastic) leukemia. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2615
Teniposide is indicated as a single agent or in combination for therapy of refractory non-Hodgkin's lymphoma. /NOT included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2615
Teniposide is indicated as a single agent or in combination for therapy of refractory neuroblastoma. /NOT included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2615
Since patients with Down's syndrome and leukemia may be particularly sensitive to myelosuppressive chemotherapy, initial dosage of teniposide should be reduced in such patients.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 1139
There currently is insufficient experience with teniposide therapy in patients with impaired renal and/or hepatic function to make specific recommendations for dosage adjustment. However, the possibility that adjustment in teniposide dosage may be necessary in such patients should be considered.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 649
The major and dose-limiting adverse effect of teniposide is hematologic toxicity. Myelosuppression, which is dose related, can be severe when teniposide is used in combination with other chemotherapeutic agents for the treatment of acute lymphocytic leukemia (ALL). Early onset of profound myelosuppression with delayed recovery can be expected when using the doses and schedules of teniposide necessary for the treatment of refractory ALL, since bone marrow hypoplasia is a desired endpoint of therapy. Severe myelosuppression with resulting infection and bleeding may occur in patients receiving the drug. Infection and bleeding have occurred in about 12 and 5%, respectively, of pediatric patients receiving teniposide monotherapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 1138
Pregnancy risk category: D /POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective./
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2616
For more Drug Warnings (Complete) data for TENIPOSIDE (18 total), please visit the HSDB record page.
Teniposide is used for the treatment of refractory acute lymphoblastic leukaemia
Teniposide is a phase-specific cytotoxic drug, acting in the late S or early G 2 phase of the cell cycle. Teniposide prevents cell mitosis by causing single and double stranded DNA breaks as well as cross linking between protein and DNA.
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01C - Plant alkaloids and other natural products
L01CB - Podophyllotoxin derivatives
L01CB02 - Teniposide
Route of Elimination
From 4% to 12% of a dose is excreted in urine as parent drug. Fecal excretion of radioactivity within 72 hours after dosing accounted for 0% to 10% of the dose.
Clearance
10.3 mL/min/m2
Teniposide was detected in intracerebral tumors at concentrations of 0.05-1.12 ug/g tissue in 11 patients given 100-150 mg/sq m teniposide 1.5-3 hr before tumor resection. The concentrations in adjacent normal brain tissue were low (< 0.9 ug/g tissue) in three patients and undetectable (< 0.05 ug/g tissue) in the others
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 267 (2000)
Teniposide was detected in one patient who died three days after a cumulative intravenous dose of 576 mg, the highest concentrations occurring in the spleen, prostate, heart, large bowel, liver and pancreas.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 267 (2000)
It is not known whether teniposide is distributed into breast milk.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2616
Elimination: Renal: 4 to 12% of a dose as unchanged teniposide. In a study of tritium-labeled teniposide in adults, 44% of the radiolabel (parent compound and metabolites) was recovered in urine within 120 hours after dosing. Fecal: 0 to 10% of a dose.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2616
For more Absorption, Distribution and Excretion (Complete) data for TENIPOSIDE (6 total), please visit the HSDB record page.
In isolated human liver preparations, cytochrome P450 mixed-function isozymes catalysed metabolism of the (pendant) E-ring to O-demethylated and catechol metabolites. This metabolism was subsequently attributed primarily to CYP3A4 activity and to a lesser degree to CYP3A5. Peroxidase-mediated O-demethylation of teniposide has also been reported.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 269 (2000)
In children given teniposide, the main metabolite in serum and urine was reported to be the hydroxy acid, formed by opening of the lactone ring; the cis-isomer, which may be a degradation product formed during storage, was also detected. The aglycone, formed by loss of the glucopyranoside moiety, was not detected (Evans et al., 1982). The hydroxy acid has not been found in plasma or urine in other studies with high doses of teniposide, and no changes in the measured concentration of teniposide in these samples was found after incubation with glucuronidase, indicating formation of little or none of the proposed glucuronide metabolites (Holthuis et al., 1987). In another study, however, 6% of the administered dose of teniposide was excreted in the urine as parent drug over 24 h, and a further 8% as a proposed aglycone glucuronide, which was not formally identified.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 267 (2000)
In general, the effects of teniposide in mammalian cells in vitro occurred in the absence of exogenous metabolic activation. Various metabolic species of teniposide have been identified, but their mutagenic properties have not been studied.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 277 (2000)
Teniposide has known human metabolites that include Teniposide catechol.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
5 hours
Terminal half life: 5 hours. NOTE: Plasma teniposide concentrations decline biexponentially following intravenous infusion.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2616
It has a multiphasic pattern of clearance from plasma. After distribution, half lives of 4 hours and 10 to 40 hours are observed.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1422
The mechanism of action appears to be related to the inhibition of type II topoisomerase activity since teniposide does not intercalate into DNA or bind strongly to DNA. Teniposide binds to and inhibits DNA topoisomerase II. The cytotoxic effects of teniposide are related to the relative number of double-stranded DNA breaks produced in cells, which are a reflection of the stabilization of a topoisomerase II-DNA intermediate.
It is an inhibitor of DNA topoisomerase II enzymes: Teniposide is a DNA topoisomerase II poison that has been shown to promote DNA cleavage, with a strong preference for a C or T at position -1. Most of the mutational events reported in mammalian cells, including point mutations, chromosomal deletions and exchanges and aneuploidy, can be explained by this activity. Teniposide does not inhibit bacterial topoisomerases and may not mutate bacterial cells by the same mechanism as mammalian cells. Unlike many other DNA topoisomerase II poisons, teniposide does not bind to DNA, either covalently or by intercalation. Instead, it appears to interact directly with the DNA topoisomerase II enzyme.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 277 (2000)
... The drug appears to produce its cytotoxic effects by damaging DNA and thereby inhibiting or altering DNA synthesis. Teniposide has been shown to induce single-stranded DNA breaks; the drug also induces double-stranded DNA breaks and DNA-protein cross links. ... Teniposide appears to be cell cycle specific, inducing G2-phase arrest and preferentially killing cells in the G2 and late S phases.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 1139
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
18
PharmaCompass offers a list of Teniposide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Teniposide manufacturer or Teniposide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Teniposide manufacturer or Teniposide supplier.
PharmaCompass also assists you with knowing the Teniposide API Price utilized in the formulation of products. Teniposide API Price is not always fixed or binding as the Teniposide Price is obtained through a variety of data sources. The Teniposide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Teniposide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Teniposide, including repackagers and relabelers. The FDA regulates Teniposide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Teniposide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Teniposide supplier is an individual or a company that provides Teniposide active pharmaceutical ingredient (API) or Teniposide finished formulations upon request. The Teniposide suppliers may include Teniposide API manufacturers, exporters, distributors and traders.
click here to find a list of Teniposide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Teniposide DMF (Drug Master File) is a document detailing the whole manufacturing process of Teniposide active pharmaceutical ingredient (API) in detail. Different forms of Teniposide DMFs exist exist since differing nations have different regulations, such as Teniposide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Teniposide DMF submitted to regulatory agencies in the US is known as a USDMF. Teniposide USDMF includes data on Teniposide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Teniposide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Teniposide suppliers with USDMF on PharmaCompass.
Teniposide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Teniposide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Teniposide GMP manufacturer or Teniposide GMP API supplier for your needs.
A Teniposide CoA (Certificate of Analysis) is a formal document that attests to Teniposide's compliance with Teniposide specifications and serves as a tool for batch-level quality control.
Teniposide CoA mostly includes findings from lab analyses of a specific batch. For each Teniposide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Teniposide may be tested according to a variety of international standards, such as European Pharmacopoeia (Teniposide EP), Teniposide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Teniposide USP).
