
Synopsis
0
CEP/COS
0
KDMF
0
NDC API
0
VMF
0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
0
Data Compilation #PharmaFlow
0
Weekly News Recap #Phispers
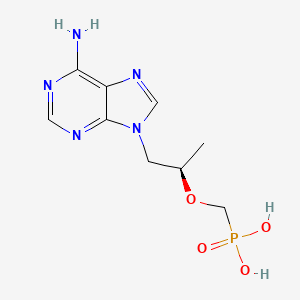

1. (r)-9-(2-phosphonylmethoxypropyl)adenine
2. 9-(2-phosphonomethoxypropyl)adenine
3. 9-(2-phosphonylmethoxypropyl)adenine
4. 9-(2-phosphonylmethoxypropyl)adenine, (+-)-isomer
5. 9-(2-phosphonylmethoxypropyl)adenine, (r)-isomer - T357098
6. 9-(2-phosphonylmethoxypropyl)adenine, (s)-isomer
7. 9-pmpa (tenofovir)
8. Disoproxil Fumarate, Tenofovir
9. Disoproxil, Tenofovir
10. Fumarate, Tenofovir Disoproxil
11. Tenofovir Disoproxil
12. Tenofovir Disoproxil Fumarate
13. Viread
1. 147127-20-6
2. (r)-9-(2-phosphonomethoxypropyl)adenine
3. Truvada
4. (r)-pmpa
5. Tenofovir (anhydrous)
6. Pmpa Gel
7. (r)-(((1-(6-amino-9h-purin-9-yl)propan-2-yl)oxy)methyl)phosphonic Acid
8. Apropovir
9. Gs-1275
10. Anhydrous Tenofovir
11. Tenofovir Anhydrous
12. Tenofovir (anh.)
13. Chebi:63625
14. Pmp-a
15. [(2r)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethylphosphonic Acid
16. ({[(2r)-1-(6-amino-9h-purin-9-yl)propan-2-yl]oxy}methyl)phosphonic Acid
17. W4hfe001u5
18. (r)-(1-(6-amino-9h-purin-9-yl)propan-2-yloxy)methylphosphonic Acid
19. 147127-20-6 (tenofovir)
20. Gs-1278
21. 9-[(r)-2-(phosphonomethoxy)propyl]adenine
22. Phosphonic Acid, P-[[(1r)-2-(6-amino-9h-purin-9-yl)-1-methylethoxy]methyl]-
23. D,l-tenofovir
24. Tenofovir Gel
25. [(1r)-2-(6-aminopurin-9-yl)-1-methyl-ethoxy]methylphosphonic Acid
26. (((1r)-2-(6-amino-9h-purin-9-yl)-1-methylethoxy)methyl)phosphonic Acid
27. (r)-[[2-(6-amino-9h-purin-9-yl)-1-methylethoxy]methyl]phosphonic Acid
28. {[(1r)-2-(6-amino-9h-purin-9-yl)-1-methylethoxy]methyl}phosphonic Acid
29. Gna & Tenofovir
30. Hha & Tenofovir
31. Tfv Gel
32. Gs 1275
33. Pmpa-(r)
34. Unii-w4hfe001u5
35. Tenofovir [usan:inn:ban]
36. Rac Tenofovir
37. Anh. Tenofovir
38. Viread (prodrug For Tenofovir)
39. Phosphonic Acid, (((1r)-2-(6-amino-9h-purin-9-yl)-1-methylethoxy)methyl)-
40. Phosphonic Acid, [[(1r)-2-(6-amino-9h-purin-9-yl)-1-methylethoxy]methyl]-
41. Phosphonic Acid, P-(((1r)-2-(6-amino-9h-purin-9-yl)-1-methylethoxy)methyl)-
42. Tenofovir- Bio-x
43. Tenofovir (viread)
44. (r)-9-(phosphonomethoxypropyl)adenine
45. Gs 1278
46. Ks-5021
47. Tenofovir [inn]
48. Tenofovir [mi]
49. 9-pmpa
50. Chembl483
51. Ec 604-571-2
52. Tenofovir [who-dd]
53. Schembl39724
54. Tenofovir Gel (gs-1278)
55. Dtxsid9040132
56. Tenofovir, >=98% (hplc)
57. Gtpl10948
58. Bcpp000049
59. Hms3264h05
60. Zinc1543475
61. Ac-760
62. Bdbm50450813
63. Mfcd00943794
64. Akos015856701
65. Akos015894941
66. Ccg-267345
67. Cs-1609
68. Db14126
69. Phosphonic Acid, ((2-(6-amino-9h-purin-9-yl)-1-methylethoxy)methyl)-, (r)-
70. Ncgc00167535-16
71. Bt164455
72. Hy-13910
73. (r)-9-(2-phosphonoylmethoxypropyl)adenine
74. Am20090678
75. T3006
76. (r)-9-[2-(phosphonomethoxy) Propyl] Adenine
77. C17407
78. Ab01274787-01
79. Ab01274787_02
80. Ab01274787_03
81. 127t206
82. A808613
83. Q155954
84. Sr-01000883934
85. Q-201787
86. Sr-01000883934-1
87. Brd-k15891719-001-02-8
88. [(2r)-3-(6-aminopurin-9-yl)-2-methyl-propyl] Dihydrogen Phosphate
89. [[(1r)-2(6-amino-9h-purin-9-yl)-1-methylethoxy]methyl]phosphonic Acid
90. [2-(6-amino-9h-purin-9-yl)-1-methylethoxy]methylphosphonic Acid
91. Phosphonic Acid, [[(1r)-2-(6-amino-9h-purin-9-yl)-1-methylethoxy]methyl]- & Galanthus Nivalis Agglutinin (gna)
92. Phosphonic Acid, [[(1r)-2-(6-amino-9h-purin-9-yl)-1-methylethoxy]methyl]- & Hippeastrum Hybrid Agglutinin( Hha)
| Molecular Weight | 287.21 g/mol |
|---|---|
| Molecular Formula | C9H14N5O4P |
| XLogP3 | -1.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 287.07834094 g/mol |
| Monoisotopic Mass | 287.07834094 g/mol |
| Topological Polar Surface Area | 136 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 354 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Tenofovir has been shown to be effective against HIV, herpes simplex virus-2, and hepatitis B virus. To know more about the specific product indications, please visit the information in the orally available forms of tenofovir, [tenofovir alafenamide] and [tenofovir disoproxil].
Treatment of HIV-1 infection:
- Truvada is indicated in antiretroviral combination therapy for the treatment of HIV-1 infected adults.
- Truvada is also indicated for the treatment of HIV-1 infected adolescents, with NRTI resistance or toxicities precluding the use of first line agents, aged 12 to < 18 years.
Pre-exposure prophylaxis (PrEP):
- Truvada is indicated in combination with safer sex practices for pre-exposure prophylaxis to reduce the risk of sexually acquired HIV-1 infection in adults at high risk.
Tenofovir has been shown to be highly effective in patients that have never had an antiretroviral therapy and it seemed to have lower toxicity than other antivirals such as [stavudine]. In phase 3 clinical trials, tenofovir presented a similar efficacy than [efavirenz] in treatment-naive HIV patients. In hepatitis B infected patients, after one year of tenofovir treatment, the viral DNA levels were undetectable.
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
Reverse Transcriptase Inhibitors
Inhibitors of reverse transcriptase (RNA-DIRECTED DNA POLYMERASE), an enzyme that synthesizes DNA on an RNA template. (See all compounds classified as Reverse Transcriptase Inhibitors.)
J05AR03
Absorption
Tenofovir as the active moiety presents a very low bioavailability when orally administered. Hence, the administration of this active agent is required to be under its two prodrug forms, [tenofovir disoproxil] and [tenofovir alafenamide]. This reduced absorption is suggested to be related to the presence of two negative charges among its structure. This negative charge limits its cellular penetration, and its passive diffusion across cellular membranes and intestinal mucosa hindering its availability for oral administration. Intravenous tenofovir has been shown to produce a maximum plasma concentration of 2500 ng/ml with an AUC of 4800 ng.h/ml.
Route of Elimination
Tenofovir is eliminated in the urine by tubular secretion and glomerular filtration. The elimination of this compound is driven by the activity of the human organic anion transporters 1 and 3 and its secretion is mainly ruled by the activity of the multidrug resistance-associated protein 4.
Volume of Distribution
Accumulation of tenofovir in plasma is related to the presence of nephrotoxic effects. It is reported that tenofovir presents a volume of distribution of 0.813 L/kg.
Clearance
The clearance of tenofovir is highly dependent on the patient renal stage and hence the clearance rate in patients with renal impairment is reported to be of 134 ml/min while in patients with normal function the clearance rate can be of 210 ml/min.
Tenofovir activation is performed by a bi-phosphorylation which in order forms the biologically active compound, tenofovir biphosphate. This metabolic activation has been shown to be performed in hepG2 cells and human hepatocytes.
The reported half-life of tenofovir is of 32 hours.
Once tenofovir is activated by a bi-phosphorylation it acts as an antiviral acyclic nucleoside phosphonate. It is a potent inhibitor of the viral reverse transcriptase with an inhibitory constant of approximately 0.022 micromolar. Once activated, tenofovir acts with different mechanisms including the inhibition of viral polymerase causing chain termination and the inhibition of viral synthesis. All these activities are attained by its competition with deoxyadenosine 5'-triphosphate in the generation of new viral DNA. Once tenofovir is incorporated in the chain, it induces a chain termination which in order inhibits viral replication. The safety of tenofovir relies on its low affinity towards the cellular DNA polymerase including the mitochondrial DNA polymerase gamma.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
53
PharmaCompass offers a list of Tenofovir API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Tenofovir manufacturer or Tenofovir supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Tenofovir manufacturer or Tenofovir supplier.
PharmaCompass also assists you with knowing the Tenofovir API Price utilized in the formulation of products. Tenofovir API Price is not always fixed or binding as the Tenofovir Price is obtained through a variety of data sources. The Tenofovir Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tenofovir manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tenofovir, including repackagers and relabelers. The FDA regulates Tenofovir manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tenofovir API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Tenofovir manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Tenofovir supplier is an individual or a company that provides Tenofovir active pharmaceutical ingredient (API) or Tenofovir finished formulations upon request. The Tenofovir suppliers may include Tenofovir API manufacturers, exporters, distributors and traders.
click here to find a list of Tenofovir suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Tenofovir DMF (Drug Master File) is a document detailing the whole manufacturing process of Tenofovir active pharmaceutical ingredient (API) in detail. Different forms of Tenofovir DMFs exist exist since differing nations have different regulations, such as Tenofovir USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Tenofovir DMF submitted to regulatory agencies in the US is known as a USDMF. Tenofovir USDMF includes data on Tenofovir's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Tenofovir USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Tenofovir suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Tenofovir Drug Master File in Japan (Tenofovir JDMF) empowers Tenofovir API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Tenofovir JDMF during the approval evaluation for pharmaceutical products. At the time of Tenofovir JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Tenofovir suppliers with JDMF on PharmaCompass.
A Tenofovir written confirmation (Tenofovir WC) is an official document issued by a regulatory agency to a Tenofovir manufacturer, verifying that the manufacturing facility of a Tenofovir active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Tenofovir APIs or Tenofovir finished pharmaceutical products to another nation, regulatory agencies frequently require a Tenofovir WC (written confirmation) as part of the regulatory process.
click here to find a list of Tenofovir suppliers with Written Confirmation (WC) on PharmaCompass.
Tenofovir Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tenofovir GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tenofovir GMP manufacturer or Tenofovir GMP API supplier for your needs.
A Tenofovir CoA (Certificate of Analysis) is a formal document that attests to Tenofovir's compliance with Tenofovir specifications and serves as a tool for batch-level quality control.
Tenofovir CoA mostly includes findings from lab analyses of a specific batch. For each Tenofovir CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tenofovir may be tested according to a variety of international standards, such as European Pharmacopoeia (Tenofovir EP), Tenofovir JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tenofovir USP).
