
Synopsis
Synopsis
0
JDMF
0
VMF
Annual Reports
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
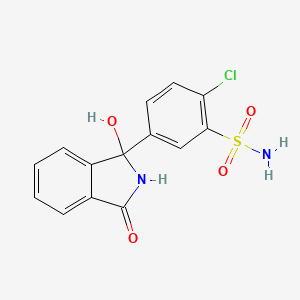

1. Chlorphthalidolone
2. Chlortalidone
3. Hygroton
4. Oxodoline
5. Phthalamudine
6. Thalitone
1. 77-36-1
2. Chlortalidone
3. Phthalamudine
4. Chlorphthalidolone
5. Hygroton
6. Chlorthalidon
7. Phthalamodine
8. Natriuran
9. Thalitone
10. Chlorothalidone
11. Chlorphthalidone
12. Saluretin
13. Zambesil
14. Igroton
15. Isoren
16. Oradil
17. Renon
18. Hydro-long
19. Chlortalidonum
20. Oxodolin
21. Oksodolin (oxodolin)
22. Racemic Chlorthalidone
23. 2-chloro-5-(1-hydroxy-3-oxoisoindolin-1-yl)benzenesulfonamide
24. Demi-regroton
25. G-33182
26. 2-chloro-5-(1-hydroxy-3-oxo-1-isoindolinyl)benzenesulfonamide
27. 1-keto-3-(3'-sulfamyl-4'-chlorophenyl)-3-hydroxyisoindoline
28. 3-hydroxy-3-(4-chloro-3-sulfamylphenyl)phthalimidine
29. 2-chloro-5-(1-hydroxy-3-oxo-2h-isoindol-1-yl)benzenesulfonamide
30. 3-(4'-chloro-3'-sulfamoylphenyl)-3-hydroxyphthalimidine
31. 1-oxo-3-(3-sulfamyl-4-chlorophenyl)-3-hydroxyisoindoline
32. G 33182
33. Nsc-69200
34. Benzenesulfonamide, 2-chloro-5-(2,3-dihydro-1-hydroxy-3-oxo-1h-isoindol-1-yl)-
35. Chlortalidone [inn]
36. Benzenesulfonamide, 2-chloro-5-(1-hydroxy-3-oxo-1-isoindolinyl)-
37. 2-chloro-5-(1-hydroxy-3-oxo-2,3-dihydro-1h-isoindol-1-yl)benzenesulfonamide
38. Mls000069531
39. Chebi:3654
40. Q0mqd1073q
41. Clortalidone
42. Famolin
43. Higroton
44. Urolin
45. 2-chloro-5-(2,3-dihydro-1-hydroxy-3-oxo-1h-isoindol-1-yl)benzenesulfonamide
46. Clortalidona
47. Smr000058635
48. (+-)-hygroton
49. Clortalidone [dcit]
50. (+-)-chlorthalidone
51. Dsstox_cid_2812
52. 2-chloro-5-(1-hydroxy-3-oxo-2,3-dihydro-1h-isoindol-1-yl)benzene-1-sulfonamide
53. Dsstox_rid_76738
54. Dsstox_gsid_22812
55. Chlortalidonum [inn-latin]
56. Clortalidona [inn-spanish]
57. Thalitone (tn)
58. Hygroton (tn)
59. Chlorthalidone (usp)
60. Hsdb 3035
61. Sr-01000721929
62. Einecs 201-022-5
63. Nsc 69200
64. Chlortalidone (jan/inn)
65. Brn 0312295
66. Chlorthalidone [usan:usp]
67. Unii-q0mqd1073q
68. Oksodolin
69. Cas-77-36-1
70. Ncgc00016317-01
71. Prestwick_759
72. Regroton (salt/mix)
73. Combipres (salt/mix)
74. Spectrum_000146
75. Opera_id_138
76. (.+/-.)-hygroton
77. Prestwick0_000351
78. Prestwick1_000351
79. Prestwick2_000351
80. Prestwick3_000351
81. Spectrum2_000099
82. Spectrum3_000349
83. Spectrum4_000957
84. Spectrum5_000743
85. (.+/-.)-chlorthalidone
86. Chlortalidone [jan]
87. Chlorthalidone [mi]
88. Chembl1055
89. Schembl26234
90. Bspbio_000441
91. Bspbio_002017
92. Chlorthalidone [hsdb]
93. Chlorthalidone [usan]
94. Kbiogr_001574
95. Kbioss_000626
96. 5-22-07-00602 (beilstein Handbook Reference)
97. Chlortalidone [mart.]
98. Chlorthalidone (chlortalidone)
99. Chlorthalidone [vandf]
100. Divk1c_000731
101. Spectrum1500187
102. Spbio_000058
103. Spbio_002362
104. Chlortalidone [who-dd]
105. Chlortalidone [who-ip]
106. Bpbio1_000487
107. Gtpl7147
108. Chlorthalidone [usp-rs]
109. Dtxsid4022812
110. Bdbm25900
111. Hms502e13
112. Kbio1_000731
113. Kbio2_000626
114. Kbio2_003194
115. Kbio2_005762
116. Kbio3_001237
117. Ninds_000731
118. Hms1569g03
119. Hms1920m09
120. Hms2091e12
121. Hms2096g03
122. Hms2236d07
123. Hms3259h04
124. Hms3372a07
125. Hms3713g03
126. Pharmakon1600-01500187
127. Chlortalidone [ep Impurity]
128. Bcp27835
129. Nsc69200
130. Chlortalidone [ep Monograph]
131. Chlorthalidone [orange Book]
132. Tox21_110369
133. Ccg-38917
134. Mfcd00036257
135. Nsc756692
136. S3074
137. Stk686335
138. Chlortalidone 1.0 Mg/ml In Methanol
139. Chlortalidonum [who-ip Latin]
140. Chlorthalidone [usp Monograph]
141. Akos005599810
142. Tox21_110369_1
143. Db00310
144. Ks-1342
145. Nc00690
146. Nsc-756692
147. Clorpres Component Chlorthalidone
148. Idi1_000731
149. Kerledex Component Chlorthalidone
150. Regroton Component Chlorthalidone
151. Combipres Component Chlorthalidone
152. Ncgc00094616-01
153. Ncgc00094616-02
154. Ncgc00094616-03
155. Ncgc00094616-04
156. Ncgc00094616-07
157. Tenoretic Component Chlorthalidone
158. Ac-11367
159. Hy-15833
160. Chlorthalidone Component Of Clorpres
161. Chlorthalidone Component Of Kerledex
162. Chlorthalidone Component Of Regroton
163. Sbi-0051313.p003
164. Chlorthalidone Component Of Combipres
165. Chlorthalidone Component Of Tenoretic
166. Db-056211
167. Lopressidone Component Chlorthalidone
168. [(3,5-difluorophenyl)amino](oxo)aceticacid
169. Ab00051946
170. Demi-regroton Component Chlorthalidone
171. C76474
172. Chlorthalidone Component Of Lopressidone
173. D00272
174. Ab00051946_12
175. Chlorthalidone Component Of Demi-regroton
176. 036c257
177. A839067
178. Q425289
179. Sr-01000721929-2
180. Sr-01000721929-3
181. W-104322
182. Brd-a26384407-001-05-3
183. Brd-a26384407-001-15-2
184. 2-chloro-5-(1-hydroxy-3-oxo-1h-isoindolinyl)benzenesulfonamide
185. Chlorthalidone, European Pharmacopoeia (ep) Reference Standard
186. 2-chloro-5-(1,3-dihydroxy-1h-isoindol-1-yl)benzene-1-sulfonamide
187. Benzenesulfonamide,3-dihydro-1-hydroxy-3-oxo-1h-isoindol-1-yl)-
188. Chlorthalidone, United States Pharmacopeia (usp) Reference Standard
189. 2-chloro-5-(1-hydroxy-3-oxo-2,3-dihydro-1h-isoindol-1-yl)benzenesulfonamide #
190. 2-chloro-5-[(1s)-1-hydroxy-3-oxo-isoindolin-1-yl]benzenesulfonamide;chlorthalidone
191. Chlortalidone For Peak Identification, European Pharmacopoeia (ep) Reference Standard
192. 74658-80-3
| Molecular Weight | 338.8 g/mol |
|---|---|
| Molecular Formula | C14H11ClN2O4S |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 338.0128057 g/mol |
| Monoisotopic Mass | 338.0128057 g/mol |
| Topological Polar Surface Area | 118 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 564 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Chlorthalidone |
| PubMed Health | Chlorthalidone (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | Chlorthalidone is an oral antihypertensive/diuretic. It is a monosulfamyl diuretic that differs chemically from thiazide diuretics in that a double-ring system is incorporated in its structure. It is 2-chloro-5(1-hydroxy-3-oxo-1- isoindolinyl) benzen... |
| Active Ingredient | Chlorthalidone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 50mg |
| Market Status | Prescription |
| Company | Mylan |
| 2 of 4 | |
|---|---|
| Drug Name | Thalitone |
| PubMed Health | Chlorthalidone (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | Thalitone (chlorthalidone USP) is an antihypertensive/diuretic supplied as 15 mg tablets for oral use. It is a monosulfamyl diuretic that differs chemically from thiazide diuretics in that a double ring system is incorporated in its structure. It i... |
| Active Ingredient | Chlorthalidone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 15mg |
| Market Status | Prescription |
| Company | Citron Pharma |
| 3 of 4 | |
|---|---|
| Drug Name | Chlorthalidone |
| PubMed Health | Chlorthalidone (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | Chlorthalidone is an oral antihypertensive/diuretic. It is a monosulfamyl diuretic that differs chemically from thiazide diuretics in that a double-ring system is incorporated in its structure. It is 2-chloro-5(1-hydroxy-3-oxo-1- isoindolinyl) benzen... |
| Active Ingredient | Chlorthalidone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 50mg |
| Market Status | Prescription |
| Company | Mylan |
| 4 of 4 | |
|---|---|
| Drug Name | Thalitone |
| PubMed Health | Chlorthalidone (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | Thalitone (chlorthalidone USP) is an antihypertensive/diuretic supplied as 15 mg tablets for oral use. It is a monosulfamyl diuretic that differs chemically from thiazide diuretics in that a double ring system is incorporated in its structure. It i... |
| Active Ingredient | Chlorthalidone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 15mg |
| Market Status | Prescription |
| Company | Citron Pharma |
Antihypertensive Agents; Diuretics, Sulfamyl
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
...ORALLY EFFECTIVE DIURETIC USEFUL IN TREATMENT OF EDEMA ASSOC WITH CONGESTIVE HEART FAILURE, RENAL DISEASE, HEPATIC CIRRHOSIS, PREGNANCY, OBESITY, & PREMENSTRUAL SYNDROME. DIURETIC EFFECTS START WITHIN 2 HR AFTER ADMIN, REACH PEAK IN 6 HR, & PERSIST FOR 48 TO 72 HR.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 871
MOST OF THIAZIDES ARE GIVEN IN DIVIDED DAILY DOSES FOR TREATMENT OF HYPERTENSION, BUT SINGLE DAILY DOSE MAY BE PREFERABLE FOR MOBILIZATION OF EDEMA FLUID. ...CHLORTHALIDONE...SHOULD BE GIVEN LESS FREQUENTLY, SINCE.../IT HAS/ DURATION OF ACTION LONGER THAN 24 HR.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 832
CHLORTHALIDONE ALSO EXERTS ANTIHYPERTENSIVE EFFECT & MAY BE ADMIN WITH OTHER AGENTS, SUCH AS RESERPINE, GANGLIONIC BLOCKING AGENTS, HYDRALAZINE, & GUANETHIDINE. SINCE.../IT/ CONTAINS SULFONAMIDE GROUP, ITS PHARMACOLOGICAL ACTIONS & MANY OF ITS UNTOWARD EFFECTS ARE SIMILAR TO THOSE OF OTHER ORALLY ADMIN DIURETICS.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 871
For more Therapeutic Uses (Complete) data for CHLORTHALIDONE (11 total), please visit the HSDB record page.
CHLORTHALIDONE IS CONTRAINDICATED IN PT WITH SEVERE RENAL OR HEPATIC DISEASE. PT ON THIS DRUG SHOULD BE WATCHED CLOSELY FOR SYMPTOMS OF RENAL DAMAGE OR OF ELECTROLYTE DISTURBANCE.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 871
May suppress lactation ... /Thiazide diuretics; from table/
Young, L.Y., M.A. Koda-Kimble (eds.). Applied Therapeutics. The Clinical Use of Drugs. 6th ed. Vancouver, WA., Applied Therapeutics, Inc. 1995., p. 45-29
Many experts consider diuretics contraindicated in pregnancy except for patients with heart disease, since they do not prevent or alter course of toxemia and may decrease placental perfusion. /Chlorothiazide; from table/
Young, L.Y., M.A. Koda-Kimble (eds.). Applied Therapeutics. The Clinical Use of Drugs. 6th ed. Vancouver, WA., Applied Therapeutics, Inc. 1995., p. 45-9
Maternal Medication usually Compatible with Breast-Feeding: Chlorthalidone: Reported Sign or Symptom in Infant or Effect on Lactation: Excreted slowly. /from Table 6/
Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 140 (1994)
For more Drug Warnings (Complete) data for CHLORTHALIDONE (14 total), please visit the HSDB record page.
3. 3= MODERATELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 0.5-5 G/KG, BETWEEN 1 OZ & 1 PINT (OR 1 LB) FOR 70 KG PERSON (150 LB).
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-239
Chlorthalidone is indicated in the management of hypertension either as the sole therapeutic agent or to enhance the effect of other antihypertensive drugs in the more severe forms of hypertension. Chlorthalidone is indicated as adjunctive therapy in edema associated with congestive heart failure, hepatic cirrhosis, and corticosteroid and estrogen therapy. Chlorthalidone has also been found useful in edema due to various forms of renal dysfunction, such as nephrotic syndrome, acute glomerulonephritis, and chronic renal failure.
FDA Label
Sodium Chloride Symporter Inhibitors
Agents that inhibit SODIUM CHLORIDE SYMPORTERS. They act as DIURETICS. Excess use is associated with HYPOKALEMIA. (See all compounds classified as Sodium Chloride Symporter Inhibitors.)
Diuretics
Agents that promote the excretion of urine through their effects on kidney function. (See all compounds classified as Diuretics.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
C - Cardiovascular system
C03 - Diuretics
C03B - Low-ceiling diuretics, excl. thiazides
C03BA - Sulfonamides, plain
C03BA04 - Chlortalidone
Route of Elimination
Approximately 50% of the administered dose is excreted unmetabolized through the kidney, and excretion is characterized by biphasic elimination with a rapid phase followed by a slow secretory phase.
Volume of Distribution
Chlorthalidone has been shown to rapidly concentrate within erythrocytes and subsequently equilibrate via a slow diffusion back into the serum compartment, resulting in a large volume of distribution.
BIOCHEM STUDIES SUGGEST THAT PROLONGED DURATION OF ACTION IS DUE TO SLOW GI ABSORPTION & ENTEROHEPATIC RECIRCULATION. DRUG IS EXCRETED UNCHANGED BY KIDNEY.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 871
MOST /THIAZIDE/ COMPD ARE RAPIDLY EXCRETED WITHIN 3 TO 6 HR. /THIAZIDE COMPD/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 831
STUDY OF DOSE-DEPENDENT URINARY EXCRETION OF CHLORTHALIDONE.
FLEUREN HLJ ET AL; DOSE-DEPENDENT URINARY EXCRETION OF CHLORTHALIDONE; CLIN PHARMACOL THER 25(6) 806-812 (1979)
Liver
40-50 hours
Chlorthalidone prevents reabsorption of sodium and chloride through inhibition of the Na+/Cl- symporter in the cortical diluting segment of the ascending limb of the loop of Henle. Reduction of sodium reabsorption subsequently reduces extracellular fluid and plasma volume via an osmotic, sodium-driven diuresis. By increasing the delivery of sodium to the distal renal tubule, Chlorthalidone indirectly increases potassium excretion via the sodium-potassium exchange mechanism. The exact mechanism of chlorthalidone's anti-hypertensive effect is under debate, however, it is thought that increased diuresis results in decreased plasma and extracellular fluid volume which therefore requires decreased cardiac output and overall lowers blood pressure. Chlorthalidone has also been shown to decrease platelet aggregation and vascular permeability, as well as promote angiogenesis in vitro, which is thought to be partly the result of reductions in carbonic anhydrasedependent pathways. These pathways may play a role in chlorthalidone's cardiovascular risk reduction effects.
...ACT BY INHIBITING RENAL TUBULAR TRANSPORT OF VARIOUS IONS.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 871
Decreased plasma volume and decreased extracellular fluid volume; decreased cardiac output initially, followed by decreased total peripheral resistance with normalization of cardiac output ... /from table/
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 552
...ANTIHYPERTENSIVE EFFECT OF CHLORTHALIDONE IS THOUGHT TO BE DUE TO DECR CARDIAC OUTPUT.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 862
The exact mechanism for reduction of arterial blood pressure by diuretics is not certain. Initially the drugs decrease extracellular volume and cardiac output. However, the hypotensive effect is maintained during long-term therapy because of reduced vascular resistance ... /Diuretics/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 781
For more Mechanism of Action (Complete) data for CHLORTHALIDONE (8 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
86
PharmaCompass offers a list of Chlorthalidone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Chlorthalidone manufacturer or Chlorthalidone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Chlorthalidone manufacturer or Chlorthalidone supplier.
PharmaCompass also assists you with knowing the Chlorthalidone API Price utilized in the formulation of products. Chlorthalidone API Price is not always fixed or binding as the Chlorthalidone Price is obtained through a variety of data sources. The Chlorthalidone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A TENORETIC 100-1 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of TENORETIC 100-1, including repackagers and relabelers. The FDA regulates TENORETIC 100-1 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. TENORETIC 100-1 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of TENORETIC 100-1 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A TENORETIC 100-1 supplier is an individual or a company that provides TENORETIC 100-1 active pharmaceutical ingredient (API) or TENORETIC 100-1 finished formulations upon request. The TENORETIC 100-1 suppliers may include TENORETIC 100-1 API manufacturers, exporters, distributors and traders.
click here to find a list of TENORETIC 100-1 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A TENORETIC 100-1 DMF (Drug Master File) is a document detailing the whole manufacturing process of TENORETIC 100-1 active pharmaceutical ingredient (API) in detail. Different forms of TENORETIC 100-1 DMFs exist exist since differing nations have different regulations, such as TENORETIC 100-1 USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A TENORETIC 100-1 DMF submitted to regulatory agencies in the US is known as a USDMF. TENORETIC 100-1 USDMF includes data on TENORETIC 100-1's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The TENORETIC 100-1 USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of TENORETIC 100-1 suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a TENORETIC 100-1 Drug Master File in Korea (TENORETIC 100-1 KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of TENORETIC 100-1. The MFDS reviews the TENORETIC 100-1 KDMF as part of the drug registration process and uses the information provided in the TENORETIC 100-1 KDMF to evaluate the safety and efficacy of the drug.
After submitting a TENORETIC 100-1 KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their TENORETIC 100-1 API can apply through the Korea Drug Master File (KDMF).
click here to find a list of TENORETIC 100-1 suppliers with KDMF on PharmaCompass.
A TENORETIC 100-1 CEP of the European Pharmacopoeia monograph is often referred to as a TENORETIC 100-1 Certificate of Suitability (COS). The purpose of a TENORETIC 100-1 CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of TENORETIC 100-1 EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of TENORETIC 100-1 to their clients by showing that a TENORETIC 100-1 CEP has been issued for it. The manufacturer submits a TENORETIC 100-1 CEP (COS) as part of the market authorization procedure, and it takes on the role of a TENORETIC 100-1 CEP holder for the record. Additionally, the data presented in the TENORETIC 100-1 CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the TENORETIC 100-1 DMF.
A TENORETIC 100-1 CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. TENORETIC 100-1 CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of TENORETIC 100-1 suppliers with CEP (COS) on PharmaCompass.
A TENORETIC 100-1 written confirmation (TENORETIC 100-1 WC) is an official document issued by a regulatory agency to a TENORETIC 100-1 manufacturer, verifying that the manufacturing facility of a TENORETIC 100-1 active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting TENORETIC 100-1 APIs or TENORETIC 100-1 finished pharmaceutical products to another nation, regulatory agencies frequently require a TENORETIC 100-1 WC (written confirmation) as part of the regulatory process.
click here to find a list of TENORETIC 100-1 suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing TENORETIC 100-1 as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for TENORETIC 100-1 API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture TENORETIC 100-1 as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain TENORETIC 100-1 and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a TENORETIC 100-1 NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of TENORETIC 100-1 suppliers with NDC on PharmaCompass.
TENORETIC 100-1 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of TENORETIC 100-1 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right TENORETIC 100-1 GMP manufacturer or TENORETIC 100-1 GMP API supplier for your needs.
A TENORETIC 100-1 CoA (Certificate of Analysis) is a formal document that attests to TENORETIC 100-1's compliance with TENORETIC 100-1 specifications and serves as a tool for batch-level quality control.
TENORETIC 100-1 CoA mostly includes findings from lab analyses of a specific batch. For each TENORETIC 100-1 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
TENORETIC 100-1 may be tested according to a variety of international standards, such as European Pharmacopoeia (TENORETIC 100-1 EP), TENORETIC 100-1 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (TENORETIC 100-1 USP).
