
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
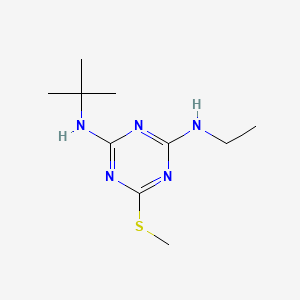

1. 2-(tert-butylamino)-4-(ethylamino)-6-(methylthio)-s-triazine
2. 2-ethylamino-6-methylthio-4-tert-butylamino-1,3, 5-triazine
3. Clarosan
4. Terbutrin
5. Terbutryne
1. 886-50-0
2. Terbutryne
3. Clarosan
4. Shortstop
5. Igran
6. Prebane
7. Terbutrex
8. Saterb
9. Igran 50
10. Short-stop E
11. N2-(tert-butyl)-n4-ethyl-6-(methylthio)-1,3,5-triazine-2,4-diamine
12. Igran 500
13. 1,3,5-triazine-2,4-diamine, N-(1,1-dimethylethyl)-n'-ethyl-6-(methylthio)-
14. Gs 14260
15. Hs-14260
16. N-(1,1-dimethylethyl)-n'-ethyl-6-(methylthio)-1,3,5-triazine-2,4-diamine
17. 1,3,5-triazine-2,4-diamine,n-(1,1-dimethylethyl)-n'-ethyl-6-(methylthio)-
18. 2-tert-butylamino-4-ethylamino-6-methylthio-1,3,5-triazine
19. A 1866
20. Zxl474tlfp
21. S-triazine, 2-(tert-butylamino)-4-(ethylamino)-6-(methylthio)-
22. 2-methylthio-4-ethylamino-6-tert-butylamino-s-triazine
23. 2-tert-butylamino-4-ethylamino-6-methylthio-s-triazine
24. 2-t-butylamino-4-ethylamino-6-methylthio-s-triazine
25. 2-tert-butylamino-4-ethylamino-6-methylmercapto-s-triazine
26. Chebi:44156
27. 4-aethylamino-2-tert-butylamino-6-methylthio-s-triazin
28. 2-tert.butylamino-4-aethylamino-6-methylthio-1,3,5-triazin
29. Dsstox_cid_4318
30. Dsstox_rid_77367
31. Dsstox_gsid_24318
32. 2-(tert-butylamino)-4-(ethylamino)-6-(methylthio)triazine
33. Caswell No. 125d
34. Terbutrin
35. N-(tert-butyl)-n'-ethyl-6-(methylthio)-1,3,5-triazine-2,4-diamine
36. N2-tert-butyl-n4-ethyl-6-(methylthio)-1,3,5-triazine-2,4-diamine
37. Terbutryne [iso-french]
38. Terbutryn [iso]
39. Cas-886-50-0
40. Terbutryn [ansi:bsi:iso]
41. Hsdb 1525
42. Einecs 212-950-5
43. Unii-zxl474tlfp
44. Epa Pesticide Chemical Code 080813
45. Brn 0611817
46. Plantonit
47. Athado
48. 4-n-tert-butyl-2-n-ethyl-6-methylsulfanyl-1,3,5-triazine-2,4-diamine
49. Terbutryn Solution
50. Amigan (salt/mix)
51. Igran 80w
52. N-tert-butyl-n-ethyl-6-methylthio-1,3,5-triazine-2,4-diamine
53. Terbutryn [hsdb]
54. 4-aethylamino-2-tert-butylamino-6-methylthio-s-triazin [german]
55. N2-tert-butyl-n4-ethyl-6-methylthio-1,3,5-triazine-2,4-diamine
56. 2-n-tert-butyl-4-n-ethyl-6-methylsulfanyl-1,3,5-triazine-2,4-diamine
57. 2-tert.butylamino-4-aethylamino-6-methylthio-1,3,5-triazin [german]
58. Oprea1_587742
59. Schembl65395
60. Mls001066371
61. Ametryn-terbutryn (salt/mix)
62. Terbutryn, Analytical Standard
63. Chembl1234490
64. Dtxsid3024318
65. Schembl20893401
66. Ex-a928
67. Hms2236m08
68. Hms3370h22
69. 2-(tert-butylamino)-4-(ethylamino)-6-(methylthio)-s-triazine
70. Hy-b1991
71. Zinc2008092
72. Tox21_202082
73. Tox21_303141
74. N2-tert-butyl-n4-ethyl-6-methylsulfanyl-1,3,5-triazine-2,4-diamine
75. Terbutryn 100 Microg/ml In Methanol
76. Akos015907183
77. Cs-5180
78. Db08215
79. Terbutryn 1000 Microg/ml In Methanol
80. Terbutryn 10 Microg/ml In Acetonitrile
81. Ncgc00164299-01
82. Ncgc00164299-02
83. Ncgc00257200-01
84. Ncgc00259631-01
85. Terbutryn 100 Microg/ml In Acetonitrile
86. As-76304
87. Smr000471867
88. Db-057093
89. Ft-0603520
90. Terbutryn, Pestanal(r), Analytical Standard
91. Butylamino-4-ethylamino-6-methylthio-s-triazine
92. C18811
93. 886t500
94. A842816
95. Methylthio-4-ethylamino-6-tert-butylamino-s-triazine
96. Q2404338
97. W-100395
98. 2-tert-butylamino-4-ethylamino-6-methylthio-[1,3,5]triazine
99. 2-tert.-butylamino-4-ethylamino-6-methylthio-[1,3,5]triazin
100. 2-tert.-butylamino-4-ethylamino-6-methylthio-[1,3,5]triazine
101. 2,4-diamine-n-(1,1-dimethylethyl)-n'-ethyl-6-(methylthio)triazine,
102. N(2)-tert-butyl-n(4)-ethyl-6-methylthio-1,3,5-triazine-2,4-diamine
103. N-(tert-butyl)-n'-ethyl-6-(methylsulfanyl)-1,3,5-triazine-2,4-diamine
104. N-tert-butyl-n'-ethyl-6-(methylsulfanyl)-1,3,5-triazine-2,4-diamine
105. N(sup 2)-tert-butyl-n(sup 4)-ethyl-6-methylthio-1,3,5-triazine-2,4-diamine
106. Terbutryn Solution, 100 Mug/ml In Acetonitrile, Pestanal(r), Analytical Standard
| Molecular Weight | 241.36 g/mol |
|---|---|
| Molecular Formula | C10H19N5S |
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 241.13611680 g/mol |
| Monoisotopic Mass | 241.13611680 g/mol |
| Topological Polar Surface Area | 88 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 206 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Herbicides
Pesticides used to destroy unwanted vegetation, especially various types of weeds, grasses (POACEAE), and woody plants. Some plants develop HERBICIDE RESISTANCE. (See all compounds classified as Herbicides.)
They are efficiently absorbed from intestine, and presumably there is some absorption across the skin and lung. /Urea-, uracil- and triazine-based herbicides/
Morgan, D. P. Recognition and Management of Pesticide Poisonings. 2nd ed. EPA 540/9-76-011, Washington, DC: U.S. Government Printing Office, Aug. 1976., p. 28
Absorbed through both foliage and roots. It appears to penetrate foliage rapidly, minimizing removal from foliage by rain. /It is/ translocated acropetally through xylem from roots and foliage, accumulating in apical meristems.
Weed Science Society of America. Herbicide Handbook. 5th ed. Champaign, Illinois: Weed Science Society of America, 1983., p. 459
In mammals, following oral admin, 73-85% is eliminated in metabolized form in feces within 24 hr.
Hartley, D. and H. Kidd (eds.). The Agrochemicals Handbook. 2nd ed. Lechworth, Herts, England: The Royal Society of Chemistry, 1987., p. A384/Aug 87
Terbutryn ... was metabolized by both rats and goats after a single oral dose by one or more of the following pathways: S-demethylation, conversion of thiomethyl into hydroxyl, N-de-ethylation, oxidation of the terminal carbon of the ethyl group to a carboxylic acid, oxidation of a terminal carbon of the t-butyl group to an alcohol or a carboxylic acid, or conjugation with glucuronic acid.
The Royal Society of Chemistry. Foreign Compound Metabolism in Mammals. Volume 6: A Review of the Literature Published during 1978 and 1979. London: The Royal Society of Chemistry, 1981., p. 323
Carbon-labeled terbutryn was admin as single oral doses to rats and goats. Urine was collected at intervals up to 72 hr and then analyzed ... after isolation of glucuronides by chromatographic procedures. Five conjugates isolated and identified were: 2-amino-4-(t-butylamino)-6-(S-glucuronyl)-s-triazine; 2-(t-butylamino)-4-ethylamino-6-(S-glucuronyl)-s-triazine; 2-ethyl-amino-(2-methyl)glucuronylpropyl)amino-6-(S-methylthio)-s-triazine; 2-amino-4-(2-(1-glucuronyl-2-methylpropyl)amino)-6-methylthio-s-triazine; 2-ethylamino-4-(2-(2-methyl propan-1-olyl)amino)-6-(S-glucuronyl)-s-triazine.
Menzie, C.M. Metabolism of Pesticides-Update III. Special Scientific Report- Wildlife No. 232. Washington, DC: U.S.Department of the Interior, Fish and Wildlife Service, 1980., p. 540
After administration of terbutryne to rats, urinary metabolites observed ... included: 2-hydroxy terbutryne; 2-amino-4-hydroxy-6-t-butylamino-s-triazine; 2-amino-4-t-butylamino-6-mercapto-s-triazine; two S-glucuronides and two t-butyl-O-glucuronides. Other metabolites were formed by one or a combination of the following reactions: N-alkyl oxidation to alcohols or acids: S-demethylation; N-deethylation; and disulfide formation.
Menzie, C.M. Metabolism of Pesticides-Update III. Special Scientific Report- Wildlife No. 232. Washington, DC: U.S.Department of the Interior, Fish and Wildlife Service, 1980., p. 540
Microsomes prepared from livers from 30 to 70 year old patients undergoing liver resection were incubated with 6.3 to 1,000 uM atrazine, terbuthylazine, terbutryne, or ametryne , and the incubation mixtures were analyzed for metabolites. The compounds produced a variety of metabolites indicative of S-oxidation, N-dealkylation, and side chain C-oxidation. The metabolites were formed by processes showing biphasic kinetics, Michaelis constants for the first and second phases varying from 1.4 to 20 uM and from 54 to 530 uM, respectively. Atrazine, terbuthylazine, ametryne, or terbutryne at 25 uM was incubated with human liver microsomes containing substrates for cytochrome-P4501A2 (CYP1A2), cytochrome-P4502A6, cytochrome-P4502D6, cytochrome-P4502C9, cytochrome-P4502C19, cytochrome-P4502E1, or cytochrome-P4503A4 (CYP3A4) isozymes. Other microsomal preparations were incubated with 25 or 600 uM of the S-triazines in the presence or absence of alpha-naphthoflavone (aNF), furafylline, quinidine, sulfaphenazole, diethyl-dithiocarbamate, gestodene, or ketoconazole, inhibitors of various specific cytochrome-P450 (P450) isozymes, at concentrations 5 to 10 times greater than their inhibition constants. Microsomal preparations containing substrates for CYP1A2 and CYP3A4 showed the best correlation with the rates of metabolism of the S-triazines. Only aNF and furafylline, inhibitors of CYP1A2, inhibited metabolism of the S-triazines. A human liver microsomal preparation with demonstrated high levels of flavin containing monooxygenase (FMO) activity and purified recombinant human FMO-3 were incubated with ametryne and terbutryne. The extent of sulfoxidation of the two compounds was determined. No significant formation of sulfoxide metabolites was detected, indicating that the FMO system was not involved in the metabolism of S- triazines by human liver microsomes. The authors conclude that these results clearly identify CYP1A2 as the major phase-I P450 isozyme that is involved in the metabolism of S-triazines by human liver microsomes.
PMID:9305587 Lang DH et al; Chem Res Toxicol 10 (9): 1037-1044 (1997)
For more Metabolism/Metabolites (Complete) data for TERBUTRYNE (7 total), please visit the HSDB record page.
Terbutryn has known human metabolites that include 1-[[4-(tert-butylamino)-6-methylsulfanyl-1,3,5-triazin-2-yl]amino]ethanol, 2-[[4-(Ethylamino)-6-methylsulfanyl-1,3,5-triazin-2-yl]amino]-2-methylpropan-1-ol, and Terbutrynsulfoxide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
... Their chief mode of action appears to involve carbohydrate metabolism. The chlorinated s-triazines inhibit starch accumulation by blocking the prodn of sugars. Similar behavior has been shown for the methoxy & methylthio-s-triazines. It has been reported that the s-triazines affect the tricarboxylic acid cycle with activation of phospho-phenyl pyruvate-carboxylase causing the disappearance of sucrose & glyceric acid with the formation of aspartic & malic acids. /S-triazines/
American Conference of Governmental Industrial Hygienists. Documentation of the Threshold Limit Values and Biological Exposure Indices. 5th ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 1986., p. 44
Inhibition of photosynthesis by disruption of light reactions and blockade of electron transport is the mechanism of action of the 1,3,5-triazine herbicides. /1,3,5-Triazines, from table/
Klaassen, C.D. (ed). Casarett and Doull's Toxicology. The Basic Science of Poisons. 6th ed. New York, NY: McGraw-Hill, 2001., p. 791
The influence of some s-triazine herbicides on acid phosphatase and phosphodiesterase from corn (Zea mays) roots were investigated. Terbutryn stimulated both phosphatases, whereas prometryn stimulated only the phosphodiesterase. Atrazine desmetryn, prometon, and simazine inhibited acid phosphatase. No effect was exerted by ametryn. The enzyme assays and the kinetic parameters demonstrated that the interferences observed were due to an action on the synthesis of one or both enzymes rather than on the enzyme reactions. The types of the N-alkyl and the chlorine-subsitutuent groups in the structures of the s-triazines tested appear important in determing the degree of the interference.
Scarponi L; Weed Sci 34 (6): 807-10 (1986)
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?