
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
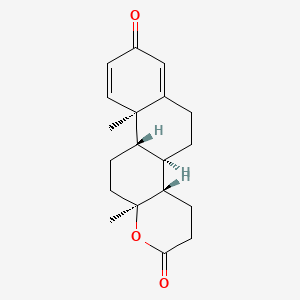

1. 1 Dehydrotestolactone
2. 1-dehydrotestolactone
3. Delta(1)-testolactone
4. Teslac
1. Teslac
2. 968-93-4
3. Teolit
4. Fludestrin
5. 1-dehydrotestololactone
6. 1,2-didehydrotestololactone
7. Delta(1)-testololactone
8. Testolattone [dcit]
9. Testolacton
10. Testolactonum [inn-latin]
11. Testolactona [inn-spanish]
12. D-homo-17a-oxaandrosta-1,4-diene-3,17-dione
13. Sq 9538
14. Sq-9538
15. Testololactone, 1-dehydro-
16. 13-hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic Acid Delta-lactone
17. Testolactone Ciii
18. Nsc-23759
19. Teslak
20. (4as,4br,10ar,10bs,12as)-10a,12a-dimethyl-3,4,4a,5,6,10a,10b,11,12,12a-decahydro-2h-naphtho[2,1-f]chromene-2,8(4bh)-dione
21. 3-oxo-13,17-secoandrosta-1,4-dieno-17,13alpha-lactone
22. 6j9bla949q
23. Chebi:9460
24. (4as,4br,10ar,10bs,12as)-10a,12a-dimethyl-4,4a,4b,5,6,10b,11,12-octahydro-3h-naphtho[2,1-f]chromene-2,8-dione
25. Testololactone, 1,2-didehydro-
26. Testolactona
27. Testolactonum
28. Testolattone
29. Nsc 23759
30. 1,2-dehydrotestololactone
31. Delta(1)-dehydrotestolactone
32. Nsc 12173
33. (4as,4br,10ar,10bs,12as)-10a,12a-dimethyl-4,4a,4b,5,6,10a,10b,11,12,12a-decahydro-2h-naphtho[2,1-f]chromene-2,8(3h)-dione
34. Delta1-testololactone
35. .delta.1-testololactone
36. Delta-1-testololactone
37. Hsdb 3255
38. .delta.1-dehydrotestolactone
39. Einecs 213-534-6
40. Teslac (tn)
41. Testolactone (usp/inn)
42. .delta.1-dehydrotestololactone
43. 17alpha-oxo-d-homo-1,4-androstadiene-3,17-dione
44. Unii-6j9bla949q
45. Nsc23759
46. Testolactone [usan:usp:inn]
47. Ncgc00159329-02
48. 1,2,3,4,4a,4b,7,9,10,10a-decahydro-2-hydroxy-2,4b-dimethyl-7-oxo-1-phenanthrenepropionic Acid Delta-lactone
49. Therapeutic Testolactone
50. Testolactone [mi]
51. Dsstox_cid_3644
52. Testolactone [inn]
53. 13,17-secoandrosta-1,4-dien-17-oic Acid, 13-hydroxy-3-oxo-, Delta-lactone
54. Testolactone [hsdb]
55. Testolactone [usan]
56. Schembl4053
57. Testololactone,2-didehydro-
58. Chembl1571
59. Dsstox_rid_77126
60. Testolactone [vandf]
61. Dsstox_gsid_23644
62. Testolactone [usp-rs]
63. Testolactone [who-dd]
64. Gtpl7303
65. Dtxsid2023644
66. Hms3750o07
67. Testolactone [orange Book]
68. Testolactone [usp Impurity]
69. Testolactone Ciii [usp-rs]
70. Bcp10926
71. Zinc4081771
72. Tox21_111576
73. Bdbm50367848
74. Lmst02020084
75. 17a-oxa-d-homoandrosta-1,17-dione
76. D-homo-17a-oxaandrosta-1,17-dione
77. Akos015840139
78. Cs-5268
79. Db00894
80. Cas-968-93-4
81. Hy-13763
82. Nci60_001908
83. C02197
84. D00153
85. 968t934
86. Q3985253
87. W-100129
88. 13,4-dien-17-oic Acid, 13-hydroxy-3-oxo-, Lactone
89. 13-hydroxy-3-oxo-13,4-dien-17-oic Acid .delta.-lactone
90. 13,4-dien-17-oic Acid, 13-hydroxy-3-oxo-, .delta.-lactone
91. 13-hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic Acid Lactone
92. 13,4-dien-17-oic Acid, 13.alpha.-hydroxy-3-oxo-, .delta.-lactone
93. 2h-phenanthro[2,8(4bh)-dione, 3,4,4a,5,6,10a,10b,11,12,12a-decahydro-10a,12a-dimethyl-, Lactone
| Molecular Weight | 300.4 g/mol |
|---|---|
| Molecular Formula | C19H24O3 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 300.17254462 g/mol |
| Monoisotopic Mass | 300.17254462 g/mol |
| Topological Polar Surface Area | 43.4 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 602 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antineoplastic Agents, Hormonal
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Testolactone has been used as adjunctive therapy in the palliative treatment of advanced or disseminated breast cancer in postmenopausal women when hormone therapy is indicated; however testolactone generally has been replaced by more effective agents. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005.
The prevalence, pathogenesis, and treatment of gynecomastia are briefly reviewed; drugs mentioned include ... testolactone.
PMID:8421478 Graustein GD; N Engl J Med 328 (Feb 18): 490-5 (1993)
The treatment of 5 girls, aged 14 months to 4.5 year, with precocious puberty in the McCune-Albright syndrome with oral testolactone tablets, ... was reported. Testolactone decreased the levels of circulating estradiol and the ovarian volume, and there was a return to pretreatment levels after testolactone was stopped. During treatment, the peak responses of luteinizing hormone and follicle-stimulating hormone to stimulation by LHRH rose above suppressed pretreatment levels (significantly above pretreatment levels for follicle-stimulating hormone) and then returned to pretreatment levels after testolactone was discontinued. Growth rates fell in 3 patients during treatment but could not be assessed in the other 2 because of bone deformities. The mean rate of bone maturation decreased and menses stopped in 3 of the 4 girls who were menstruating regularly. It was concluded that testolactone is an effective treatment of precocious puberty in the McCune-Albright syndrome.
PMID:3093862 Feuillan PP et al; N Engl J Med 315 (Oct 30): 1115-9 (1986)
For more Therapeutic Uses (Complete) data for TESTOLACTONE (8 total), please visit the HSDB record page.
Side/Adverse effects: Those indicating need for medical attention: Incidence less frequent: Peripheral neuropathies (numbness or tingling of fingers, toes, or face). Those indicating need for medical attention only if they continue or are bothersome: Incidence less frequent: Diarrhea; loss of appetite; nausea or vomiting; pain or swelling in feet or lower legs; swelling or redness of tongue.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005.
Maculopapular erythema and an increase in blood pressure have been reported rarely during testolactone therapy. Paresthesia, aches and edema of the extremities, glossitis, anorexia, hot flushes, nausea, vomiting, and diarrhea have occurred in patients receiving testolactone, but these adverse effects have not been definitely attributed to the drug. Alopecia alone or with associated nail growth disturbance has been reported rarely during testolactone therapy; however, these adverse effects subsided with continued therapy. Elevation of urinary 17-ketosteroids and creatine has occurred in patients receiving oral dosages of 150 mg of testolactone daily. Increased erythropoiesis has been reported in a patient with myeloid metaplasia receiving testolactone.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 1166
Testolactone is not recommended for treatment of breast cancer in males.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005.
Although hypercalcemia has not been reported in patients receiving testolactone to date, plasma calcium concentrations should be routinely monitored in patients receiving the drug, particularly during periods of active remission of bony metastases. If hypercalcemia occurs, appropriate therapy, including high fluid intake, should be instituted.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 1166
FDA Pregnancy Risk Category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005.
For palliative treatment of advanced breast cancer in postmenopausal women.
FDA Label
Testolactone is a synthetic anti-neoplastic agent that is structurally distinct from the androgen steroid nucleus in possessing a six-membered lactone ring in place of the usual five-membered carbocyclic D-ring. Despite some similarity to testosterone, testolactone has no in vivo androgenic effect. No other hormonal effects have been reported in clinical studies in patients receiving testolactone.
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
Absorption
Testolactone is well absorbed from the gastrointestinal tract.
Route of Elimination
No clinical effects in humans of testolactone on adrenal function have been reported; however, one study noted an increase in urinary excretion of 17-ketosteroids in most of the patients treated with 150 mg/day orally. It is metabolized to several derivatives in the liver, all of which preserve the lactone D-ring. These metabolites, as well as some unmetabolized drug, are excreted in the urine.
Elimination: Renal.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005.
It is not known whether testolactone is distributed into milk.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 1167
Well absorbed from the gastrointestinal tract.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005.
Biotransformation: Hepatic.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005.
Hepatic. Metabolized to several derivatives in the liver, all of which preserve the lactone D-ring.
Testolactone is metabolized primarily in the liver and excreted in urine. Compounds recovered in urine after testolactone administration have included 3a, 13alpha-dihydroxy-13,17-seco-5beta-androsta-1-ene-17-oic acid lactone and its glucuronide, and unchanged testolactone.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 1167
Although the precise mechanism by which testolactone produces its clinical antineoplastic effects has not been established, its principal action is reported to be inhibition of steroid aromatase activity and consequent reduction in estrone synthesis from adrenal androstenedione, the major source of estrogen in postmenopausal women. Based on in vitro studies, the aromatase inhibition may be noncompetitive and irreversible. This phenomenon may account for the persistence of testolactone's effect on estrogen synthesis after drug withdrawal.
The exact mechanism of the antineoplastic action of testolactone has not been determined; however, it has been suggested that the drug may reduce estrone synthesis from adrenal androstenedione via noncompetitive, irreversible inhibition of A-ring reductase (aromatase). Because estrogen acts as a growth factor for hormone-dependent breast cancer cells, reduction of serum and tumor concentrations of estrogen inhibits tumor growth and delays disease progression. In postmenopausal women, ovarian secretion of estrogen declines and conversion of adrenal androgens (mainly androstenedione and testosterone) to estrone and estradiol in peripheral tissues (adipose, muscle, and liver), catalyzed by the aromatase enzyme, is the principal source of estrogens. Inhibition of the aromatase enzyme by testolactone results in suppression of estrogen biosynthesis in all tissues, reducing serum concentrations of circulating estrogens, including estrone, estradiol, and estrone sulfate.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 1167
Aminoglutethimide and testolactone may be considered the first generation aromatase inhibitors for the endocrine treatment of breast carcinoma. Initially, both of these agents were designed and used clinically based on different concepts of their mechanisms of action. Only later were they both demonstrated to inhibit aromatase. Curiously, testololactone was earlier and more widely used than aminoglutethimide in treating advanced breast carcinoma. The discovery of the peripheral aromatase inhibition as the proper mechanism of action was delayed for both the agents but was relatively more timely for aminoglutethimide. Paradoxically, the clinical use of testololactone has become already obsolete since its true mechanism of action was discovered.
PMID:7949205 Cocconi G; Breast Cancer Res Treat 30 (1): 57-80 (1994)
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
26
PharmaCompass offers a list of Testolactone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Testolactone manufacturer or Testolactone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Testolactone manufacturer or Testolactone supplier.
PharmaCompass also assists you with knowing the Testolactone API Price utilized in the formulation of products. Testolactone API Price is not always fixed or binding as the Testolactone Price is obtained through a variety of data sources. The Testolactone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Testolactone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Testolactone, including repackagers and relabelers. The FDA regulates Testolactone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Testolactone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Testolactone supplier is an individual or a company that provides Testolactone active pharmaceutical ingredient (API) or Testolactone finished formulations upon request. The Testolactone suppliers may include Testolactone API manufacturers, exporters, distributors and traders.
click here to find a list of Testolactone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Testolactone DMF (Drug Master File) is a document detailing the whole manufacturing process of Testolactone active pharmaceutical ingredient (API) in detail. Different forms of Testolactone DMFs exist exist since differing nations have different regulations, such as Testolactone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Testolactone DMF submitted to regulatory agencies in the US is known as a USDMF. Testolactone USDMF includes data on Testolactone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Testolactone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Testolactone suppliers with USDMF on PharmaCompass.
Testolactone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Testolactone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Testolactone GMP manufacturer or Testolactone GMP API supplier for your needs.
A Testolactone CoA (Certificate of Analysis) is a formal document that attests to Testolactone's compliance with Testolactone specifications and serves as a tool for batch-level quality control.
Testolactone CoA mostly includes findings from lab analyses of a specific batch. For each Testolactone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Testolactone may be tested according to a variety of international standards, such as European Pharmacopoeia (Testolactone EP), Testolactone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Testolactone USP).
