
Synopsis
Synopsis
0
JDMF
0
KDMF
0
VMF
0
Australia

0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Amethocaine
2. Ametop
3. Dicaine
4. Hydrochloride, Tetrracaine
5. Pantocaine
6. Pontocaine
7. Tetracaine Monohydrochloride
8. Tetrakain
9. Tetrracaine Hydrochloride
1. 94-24-6
2. Amethocaine
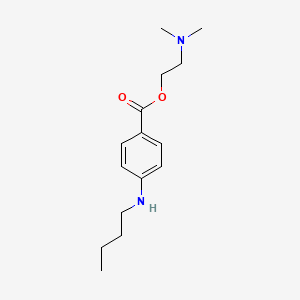
3. 2-(dimethylamino)ethyl 4-(butylamino)benzoate
4. Pontocaine
5. Dicaine
6. Laudocaine
7. Metraspray
8. Tetrakain
9. Anetain
10. Contralgin
11. Meethobalm
12. Mucaesthin
13. Tetracaina
14. Tetracainum
15. Uromucaesthin
16. Fissucain
17. Intercain
18. Medicaine
19. Niphanoid
20. Rexocaine
21. Dicain
22. Dikain
23. Medihaler-tetracaine
24. 2-(dimethylamino)ethyl P-(butylamino)benzoate
25. P-butylaminobenzoyl-2-dimethylaminoethanol
26. Benzoic Acid, 4-(butylamino)-, 2-(dimethylamino)ethyl Ester
27. Diaethylaminoaethanol Ester Der P-butylaminobenzoesaeure
28. 4-(butylamino)benzoic Acid 2-(dimethylamino)ethyl Ester
29. Dimethylaminoethyl P-butyl-aminobenzoate
30. Chebi:9468
31. P-(butylamino)benzoic Acid, 2-(dimethylamino)ethyl Ester
32. Mfcd00053787
33. Chembl698
34. Benzoic Acid, P-(butylamino)-, 2-(dimethylamino)ethyl Ester
35. 2-dimethylaminoethylester Kyseliny P-butylaminobenzoove
36. Tetracaine Base
37. 0619f35cgv
38. Butylocaine
39. Tetrakain [czech]
40. Tetracainum [inn-latin]
41. Tetracaina [inn-spanish]
42. P-(butylamino)benzoic Acid Beta-(dimethylamino)ethyl Ester
43. Amethocaine (tn)
44. Tetracaine (usp/inn)
45. Ncgc00016049-02
46. Cas-136-47-0
47. Einecs 202-316-6
48. Brn 2216051
49. Landocaine
50. Tetracaine [usp:inn:ban]
51. Unii-0619f35cgv
52. Amethocaine Hcl
53. 2-dimethylaminoethylester Kyseliny P-butylaminobenzoove [czech]
54. Diaethylaminoaethanol Ester Der P-butylaminobenzoesaeure [german]
55. Te4
56. Spectrum_001032
57. Pantocaine (salt/mix)
58. Tetracaine [inn]
59. 100311-22-6
60. Prestwick0_000571
61. Prestwick1_000571
62. Prestwick2_000571
63. Prestwick3_000571
64. Spectrum2_001328
65. Spectrum3_000562
66. Spectrum4_000351
67. Spectrum5_001072
68. Lopac-t-7508
69. Tetracaine [vandf]
70. Epitope Id:174843
71. Tetracaine [mart.]
72. Tetracaine [usp-rs]
73. Tetracaine [who-dd]
74. Lopac0_001211
75. Schembl34714
76. Bspbio_000382
77. Bspbio_001944
78. Kbiogr_000781
79. Kbioss_001512
80. 4-14-00-01172 (beilstein Handbook Reference)
81. Divk1c_000607
82. Spbio_001455
83. Spbio_002601
84. Bpbio1_000422
85. Tetracaine [green Book]
86. Tetracaine, >=98% (tlc)
87. Dtxsid1043883
88. Tetracaine [orange Book]
89. Kbio1_000607
90. Kbio2_001512
91. Kbio2_004080
92. Kbio2_006648
93. Kbio3_001444
94. Tetracaine [ep Monograph]
95. Ninds_000607
96. Synera Component Tetracaine
97. Bcpp000048
98. Tetracaine [usp Monograph]
99. Act04765
100. Albb-025902
101. Bcp04777
102. Hy-a0079
103. Zinc1530811
104. Bdbm50017659
105. Stl483844
106. Tetracaine Component Of Synera
107. Akos015889234
108. Ac-3480
109. Ccg-205285
110. Db09085
111. Sdccgsbi-0051178.p005
112. Idi1_000607
113. Ncgc00016049-01
114. Ncgc00016049-03
115. Ncgc00016049-04
116. Ncgc00016049-14
117. Ncgc00162367-01
118. As-81743
119. Sy066710
120. Bcp0726000001
121. Sbi-0051178.p004
122. Ab00053549
123. Ft-0656378
124. T2789
125. Tetracaine, Meets Usp Testing Specifications
126. .beta.-dimethylaminoethyl P-butylaminobenzoate
127. 2-(dimethylamino)ethyl4-(n-butylamino)benzoate
128. C07526
129. D00551
130. 2-(dimethylamino)ethyl 4-(butylamino)benzoate #
131. Ab00053549-11
132. Ab00053549_12
133. Ab00053549_13
134. Q419608
135. Q-201805
136. Brd-k45071273-003-05-8
137. Brd-k45071273-003-15-7
138. Tetracaine, United States Pharmacopeia (usp) Reference Standard
139. Benzoic Acid, 4-(butylamino)-, 2-(dimethylamino)ethyl Ester ,hydrochloride
140. Benzoic Acid,4-butylamino,2-dimethylaminoethyl Ester Pantocain Base
| Molecular Weight | 264.36 g/mol |
|---|---|
| Molecular Formula | C15H24N2O2 |
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 9 |
| Exact Mass | 264.183778013 g/mol |
| Monoisotopic Mass | 264.183778013 g/mol |
| Topological Polar Surface Area | 41.6 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 249 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ophthalmic tetracaine is indicated for the for procedures requiring a rapid and short- acting topical ophthalmic anesthetic. The combination lidocaine and tetracaine patch is indicated for local dermal analgesia for superficial dermatological procedures and superficial venous access. The combination lidocaine and tetracaine cream is intended to provide topical local analgesia for superficial dermatological procedures.
FDA Label
Anesthetics, Local
Drugs that block nerve conduction when applied locally to nerve tissue in appropriate concentrations. They act on any part of the nervous system and on every type of nerve fiber. In contact with a nerve trunk, these anesthetics can cause both sensory and motor paralysis in the innervated area. Their action is completely reversible. (From Gilman AG, et. al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed) Nearly all local anesthetics act by reducing the tendency of voltage-dependent sodium channels to activate. (See all compounds classified as Anesthetics, Local.)
S01HA03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C05 - Vasoprotectives
C05A - Agents for treatment of hemorrhoids and anal fissures for topical use
C05AD - Local anesthetics
C05AD02 - Tetracaine
D - Dermatologicals
D04 - Antipruritics, incl. antihistamines, anesthetics, etc.
D04A - Antipruritics, incl. antihistamines, anesthetics, etc.
D04AB - Anesthetics for topical use
D04AB06 - Tetracaine
N - Nervous system
N01 - Anesthetics
N01B - Anesthetics, local
N01BA - Esters of aminobenzoic acid
N01BA03 - Tetracaine
S - Sensory organs
S01 - Ophthalmologicals
S01H - Local anesthetics
S01HA - Local anesthetics
S01HA03 - Tetracaine
Absorption
Systemic absorption of anaesthetic from the combination cream is directly related to the duration and surface area of application. Although peak plasma concentrations for lidocaine were measured, plasma levels for tetracaine could not be determined due to low levels (<0.9 ng/mL)
Volume of Distribution
Tetracaine is rapidly hydrolyzed in the plasma; therefore, volume of distribution could not be determined.
Clearance
Tetracaine is hydrolyzed rapidly in the plasma; therefore, clearance has not been determined.
Tetracaine is rapidly hydrolyzed by plasma esterases to the following primary metabolites: para-aminobenzoic acid and diethylaminoethanol. The activity of both metabolites is unspecified.
Tetracaine is hydrolyzed rapidly in the plasma; therefore, half-life has not been determined.
Tetracaine is an ester-type anesthetic and produces local anesthesia by blocking the sodium ion channels involved in initiation and conduction of neuronal impulses.
 Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.

NDC Package Code : 58159-107
Start Marketing Date : 2024-10-21
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (35kg/35kg)
Marketing Category : BULK INGREDIENT
 China's leading API and intermediate manufacturer, offering CMO/CDMO services and exports to 70+ countries, enhancing global health.
China's leading API and intermediate manufacturer, offering CMO/CDMO services and exports to 70+ countries, enhancing global health.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35615
Submission : 2021-02-04
Status : Active
Type : II

NDC Package Code : 82481-004
Start Marketing Date : 2022-01-05
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 Apex Healthcare Limited: ISO 9001:2008, WHO-GMP, US FDA-audited manufacturer & exporter of APIs, bulk drugs, and formulations.
Apex Healthcare Limited: ISO 9001:2008, WHO-GMP, US FDA-audited manufacturer & exporter of APIs, bulk drugs, and formulations.
 SWATI - Transforming science into solutions with 60+ years of expertise, global accreditations, and pioneering biotech innovation.
SWATI - Transforming science into solutions with 60+ years of expertise, global accreditations, and pioneering biotech innovation.
 Willow Birch Pharma delivers trusted, high-quality APIs nationwide with unmatched service, compliance, and competitive value.
Willow Birch Pharma delivers trusted, high-quality APIs nationwide with unmatched service, compliance, and competitive value.

NDC Package Code : 10695-087
Start Marketing Date : 2021-07-14
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 22496
Submission : 2008-12-31
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37343
Submission : 2025-09-05
Status : Active
Type : II

Certificate Number : R0-CEP 2021-158 - Rev 00
Issue Date : 2022-01-12
Type : Chemical
Substance Number : 2909
Status : Valid

NDC Package Code : 17381-099
Start Marketing Date : 2010-05-18
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT



GDUFA
DMF Review : Reviewed
Rev. Date : 2018-10-30
Pay. Date : 2018-09-12
DMF Number : 24100
Submission : 2010-08-26
Status : Active
Type : II

Date of Issue : 2024-07-31
Valid Till : 2027-01-24
Written Confirmation Number : WC-0291
Address of the Firm :


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : PLIAGLIS
Dosage Form : CREAM
Dosage Strength : 7%
Packaging : 30G
Approval Date :
Application Number : 2398028
Regulatory Info : Prescription
Registration Country : Canada

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
75
PharmaCompass offers a list of Tetracaine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Tetracaine manufacturer or Tetracaine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Tetracaine manufacturer or Tetracaine supplier.
PharmaCompass also assists you with knowing the Tetracaine API Price utilized in the formulation of products. Tetracaine API Price is not always fixed or binding as the Tetracaine Price is obtained through a variety of data sources. The Tetracaine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tetracaine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tetracaine, including repackagers and relabelers. The FDA regulates Tetracaine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tetracaine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Tetracaine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Tetracaine supplier is an individual or a company that provides Tetracaine active pharmaceutical ingredient (API) or Tetracaine finished formulations upon request. The Tetracaine suppliers may include Tetracaine API manufacturers, exporters, distributors and traders.
click here to find a list of Tetracaine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Tetracaine DMF (Drug Master File) is a document detailing the whole manufacturing process of Tetracaine active pharmaceutical ingredient (API) in detail. Different forms of Tetracaine DMFs exist exist since differing nations have different regulations, such as Tetracaine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Tetracaine DMF submitted to regulatory agencies in the US is known as a USDMF. Tetracaine USDMF includes data on Tetracaine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Tetracaine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Tetracaine suppliers with USDMF on PharmaCompass.
A Tetracaine CEP of the European Pharmacopoeia monograph is often referred to as a Tetracaine Certificate of Suitability (COS). The purpose of a Tetracaine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Tetracaine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Tetracaine to their clients by showing that a Tetracaine CEP has been issued for it. The manufacturer submits a Tetracaine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Tetracaine CEP holder for the record. Additionally, the data presented in the Tetracaine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Tetracaine DMF.
A Tetracaine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Tetracaine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Tetracaine suppliers with CEP (COS) on PharmaCompass.
A Tetracaine written confirmation (Tetracaine WC) is an official document issued by a regulatory agency to a Tetracaine manufacturer, verifying that the manufacturing facility of a Tetracaine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Tetracaine APIs or Tetracaine finished pharmaceutical products to another nation, regulatory agencies frequently require a Tetracaine WC (written confirmation) as part of the regulatory process.
click here to find a list of Tetracaine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Tetracaine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Tetracaine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Tetracaine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Tetracaine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Tetracaine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Tetracaine suppliers with NDC on PharmaCompass.
Tetracaine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tetracaine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tetracaine GMP manufacturer or Tetracaine GMP API supplier for your needs.
A Tetracaine CoA (Certificate of Analysis) is a formal document that attests to Tetracaine's compliance with Tetracaine specifications and serves as a tool for batch-level quality control.
Tetracaine CoA mostly includes findings from lab analyses of a specific batch. For each Tetracaine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tetracaine may be tested according to a variety of international standards, such as European Pharmacopoeia (Tetracaine EP), Tetracaine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tetracaine USP).

