
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
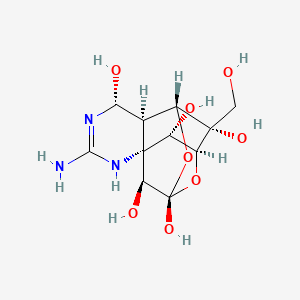

1. Fugu Toxin
2. Tarichatoxin
3. Tetradotoxin
4. Toxin, Fugu
1. Spheroidine
2. Tarichatoxin
3. Tetrodotoxine
4. Babylonia Japonica Toxin 1
5. Bjt 1
6. Maculotoxin
7. 4368-28-9
8. Tectin
9. Araregai Toxin
10. Pft-1 Toxin
11. Tetrodontoxin
12. Fugu Poison
13. Chembl507974
14. 3kum2721u9
15. Ttx
16. Tetrodoxin
17. (1r,5r,6r,7r,9s,11s,12s,13s,14s)-3-amino-14-(hydroxymethyl)-8,10-dioxa-2,4-diazatetracyclo[7.3.1.1~7,11~.0~1,6~]tetradec-3-ene-5,9,12,13,14-pentol (non-preferred Name)
18. Tettrodotoxin
19. Unii-3kum2721u9
20. Ccris 9328
21. Hsdb 3543
22. 9sr
23. Octahydro-12-(hydroxymethyl)-2-imino-5,9:7,10a-dimethano-10ah-(1,3)dioxocino(6,5-d)pyrimidine-4,7,10,11,12-pentol
24. Einecs 224-458-8
25. Brn 0049176
26. Tetrodotoxin [mi]
27. Tetrodotoxin [inn]
28. 4-27-00-08206 (beilstein Handbook Reference)
29. Tetrodotoxin [who-dd]
30. Schembl6406675
31. Bdbm50344821
32. Zinc13780673
33. (4r-(4alpha,4aalpha,5alpha,7alpha,9alpha,10alpha,10abeta,11s*,12s*))-octahydro-12-(hydroxymethyl)-2-imino-5,9:7,10a-dimethano-10ah-(1,3)dioxocino(6,5-d) Pyrimidine-4,7,10,11,12-pentol
34. 5,9:7,10a-dimethano-10ah-(1,3)dioxocino(6,5-d)pyrimidine-4,7,10,11,12-pentol, Octahydro-12-(hydroxymethyl)-2-imino-, (4r-(4alpha,4aalpha,5alpha,7alpha,9alpha,10alpha,10abeta,11s*,12s*))-
35. Q-100286
36. 10-hydroxymethyl-5-imino-(2s)-12,13-dioxa-4,6-diazatetracyclo[7.3.1.13,11.03,8]tetradecane-1,2,7,10,14-pentaol
37. 10-hydroxymethyl-5-imino-(2s)-12,13-dioxa-4,6-diazatetracyclo[7.3.1.13,11.03,8]tetradecane-1,2,7,10,14-pentaolcitrate
38. 5,9:7,10a-dimethano-10ah-(1,3)dioxocino(6,5-d)pyrimidine-4,7,10,11,12-pentol, 2-amino-1,4,4a,5,9,10-hexahydro-12-(hydroxymethyl)-, (4r,4ar,5r,7s,9s,10s,10ar,11s,12s)-
39. 5,9:7,10a-dimethano-10ah-(1,3)dioxocino(6,5-d)pyrimidine-4,7,10,11,12-pentol, Octahydro-12-(hydroxymethyl)-2-imino-
40. 5,9:7,10a-dimethano-10ah-(1,3)dioxocino(6,5-d)pyrimidine-4,7,10,11,12-pentol, Octahydro-12-(hydroxymethyl)-2-imino-, (4r,4ar,5r,7s,9s,10s,10ar,11s,12s)-
| Molecular Weight | 319.27 g/mol |
|---|---|
| Molecular Formula | C11H17N3O8 |
| XLogP3 | -5.9 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 1 |
| Exact Mass | 319.10156451 g/mol |
| Monoisotopic Mass | 319.10156451 g/mol |
| Topological Polar Surface Area | 190 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 562 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ Corneal injury can produce photophobia, an aversive sensitivity to light. Using topical application of lidocaine, a local anesthetic, and tetrodotoxin (TTX), a selective voltage-sensitive sodium channel blocker, we assessed whether enhanced aversiveness to light induced by corneal injury in rats was caused by enhanced activity in corneal afferents. Eye closure induced by 30 seconds of exposure to bright light (460-485 nm) was increased 24 hours after corneal injury induced by de-epithelialization. Although the topical application of lidocaine did not affect the baseline eye closure response to bright light in control rats, it eliminated the enhancement of the response to the light stimulus after corneal injury (photophobia). Similarly, topical application of TTX had no effect on the eye closure response to bright light in rats with intact corneas, but it markedly attenuated photophobia in rats with corneal injury. Given the well-established corneal toxicity of local anesthetics, we suggest TTX as a therapeutic option to treat photophobia and possibly other symptoms that occur in clinical diseases that involve corneal nociceptor sensitization. PERSPECTIVE: We show that lidocaine and TTX attenuate photophobia induced by corneal injury. Although corneal toxicity limits use of local anesthetics, TTX may be a safer therapeutic option to reduce the symptom of photophobia associated with corneal injury.
PMID:26086898 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4664153 Green PG et al; J Pain 16 (9): 881-6 (2015)
/EXPL THER/ Burn injuries have been identified as the primary cause of injury in 5% of U.S. military personnel evacuated from Operations Iraqi Freedom and Enduring Freedom. Severe burn-associated pain is typically treated with opioids such as fentanyl, morphine, and methadone. Side effects of opioids include respiratory depression, cardiac depression, decrease in motor and cognitive function, as well as the development of hyperalgesia, tolerance and dependence. These effects have led us to search for novel analgesics for the treatment of burn-associated pain in wounded combat service members. Tetrodotoxin (TTX) is a selective voltage-gated sodium channel blocker currently in clinical trials as an analgesic. A phase 3 clinical trial for cancer-related pain has been completed and phase 3 clinical trials on chemotherapy-induced neuropathic pain are planned. It has also been shown in mice to inhibit the development of chemotherapy-induced neuropathic pain. TTX was originally identified as a neurotoxin in marine animals but has now been shown to be safe in humans at therapeutic doses. The antinociceptive effects of TTX are thought to be due to inhibition of Na(+) ion influx required for initiation and conduction of nociceptive impulses. One TTX sensitive sodium channel, Nav1.7, has been shown to be essential in lowering the heat pain threshold after burn injuries. To date, the analgesic effect of TTX has not been tested in burn-associated pain. Male Sprague-Dawley rats were subjected to a full thickness thermal injury on the right hind paw. TTX (8 ug/kg) was administered once a day systemically by subcutaneous injection beginning 3 days post thermal injury and continued through 7 days post thermal injury. Thermal hyperalgesia and mechanical allodynia were assessed 60 and 120 min post injection on each day of TTX treatment. TTX significantly reduced thermal hyperalgesia at all days tested and had a less robust, but statistically significant suppressive effect on mechanical allodynia. These results suggest that systemic TTX may be an effective, rapidly acting analgesic for battlefield burn injuries and has the potential for replacing or reducing the need for opioid analgesics.
PMID:26424077 Salas MM et al; Neurosci Lett 607: 108-113 (2015)
/EXPL THER/ Persistent muscle pain is a common and disabling symptom for which available treatments have limited efficacy. Since tetrodotoxin (TTX) displays a marked antinociceptive effect in models of persistent cutaneous pain, we tested its local antinociceptive effect in rat models of muscle pain induced by inflammation, ergonomic injury and chemotherapy-induced neuropathy. While local injection of TTX (0.03-1 ug) into the gastrocnemius muscle did not affect the mechanical nociceptive threshold in naive rats, exposure to the inflammogen carrageenan produced a marked muscle mechanical hyperalgesia, which was dose-dependently inhibited by TTX. This antihyperalgesic effect was still significant at 24 hr. TTX also displayed a robust antinociceptive effect on eccentric exercise-induced mechanical hyperalgesia in the gastrocnemius muscle, a model of ergonomic pain. Finally, TTX produced a small but significant inhibition of neuropathic muscle pain induced by systemic administration of the cancer chemotherapeutic agent oxaliplatin. These results indicate that TTX-sensitive sodium currents in nociceptors play a central role in diverse states of skeletal muscle nociceptive sensitization, supporting the suggestion that therapeutic interventions based on TTX may prove useful in the treatment of muscle pain.
PMID:26548414 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4679288 Alvarez P, Levine JD; Neuroscience 311: 499-507 (2015)
/EXPL THER/ OBJECTIVE: This study evaluated subcutaneous injections of tetrodotoxin (TTX) for the treatment of moderate to severe, inadequately controlled cancer-related pain. METHODS: Eligible patients were randomized to receive TTX (30 ug) or placebo subcutaneously twice daily for four consecutive days. Efficacy was assessed using pain and composite endpoints (including pain and quality of life measures), and safety was evaluated using standard measures. RESULTS: 165 patients were enrolled at 19 sites in Canada, Australia, and New Zealand, with 149 patients in the primary analysis "intent-to-treat" population. The primary analysis supports a clinical benefit of TTX over placebo based on the pain endpoint alone with a clinically significant estimated effect size of 16.2% (p = 0.0460). The p value was nominally statistically significant after prespecified (Bonferroni Holm) adjustment for the two primary endpoints but not at the prespecified two-sided 5% level. The mean duration of analgesic response was 56.7 days (TTX) and 9.9 days (placebo). Most common adverse events were nausea, dizziness, and oral numbness or tingling and were generally mild to moderate and transient. CONCLUSIONS: Although underpowered, this study demonstrates a clinically important analgesic signal. TTX may provide clinically meaningful analgesia for patients who have persistent moderate to severe cancer pain despite best analgesic care.
PMID:28555092 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5438848 Hagen NA et al; Pain Res Manag 2017:7212713. doi: 10.1155/2017/7212713. Epub 2017 May 7 (2017)
For more Therapeutic Uses (Complete) data for Tetrodotoxin (6 total), please visit the HSDB record page.
A fatal dose may be as little as 1 to 4 mg per person.
Klaassen, C.D. (ed). Casarett and Doull's Toxicology. The Basic Science of Poisons. 6th ed. New York, NY: McGraw-Hill, 2001., p. 1079
... the minimum lethal dose in an adult human is estimated to be 2-3 mg.
PMID:25551594 Cole JB et al; MMWR Morb Mortal Wkly Rep 63 (51): 1222-5 (2015)
Anesthetics, Local
Drugs that block nerve conduction when applied locally to nerve tissue in appropriate concentrations. They act on any part of the nervous system and on every type of nerve fiber. In contact with a nerve trunk, these anesthetics can cause both sensory and motor paralysis in the innervated area. Their action is completely reversible. (From Gilman AG, et. al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed) Nearly all local anesthetics act by reducing the tendency of voltage-dependent sodium channels to activate. (See all compounds classified as Anesthetics, Local.)
Poisons
Substances which, when ingested, inhaled, or absorbed, or when applied to, injected into, or developed within the body in relatively small amounts may, by their chemical action, cause damage to structure or disturbance of function. (From Dorland, 27th ed) (See all compounds classified as Poisons.)
Sodium Channel Blockers
A class of drugs that act by inhibition of sodium influx through cell membranes. Blockade of sodium channels slows the rate and amplitude of initial rapid depolarization, reduces cell excitability, and reduces conduction velocity. (See all compounds classified as Sodium Channel Blockers.)
Twenty-three specimens of a tree-frog Polypedates sp. were collected from two locations (Mymensingh and Barisal) of Bangladesh in 1999, and assayed for their toxicity scores and toxin principle. Among the tissues, only the skin of the Mymensingh specimens was found to be toxic in mouse test, with the toxicity scores of 31-923 ug/g. The toxin isolated from the skin was analyzed by high-performance liquid chromatography, electrospray ionization-time of flight mass spectrometry and proton nuclear magnetic resonance, and characterized as tetrodotoxin, a toxin principle.
PMID:11223081 Tanu MB et al; Toxicon 39 (7): 937-41 (2001)
Tetrodotoxin (TTX) and its analogs (TTXs), widely distributed among marine as well as terrestrial animals, induce dangerous intoxications. These highly potential toxins are also known as the causative agent of puffer fish poisoning. ... TTX, anhydrotetrodotoxin, 11-deoxytetrodotoxin and trideoxytetrodotoxin were determined in separated tissues of Bangladeshi marine puffers, Takifugu oblongus. TTX was predominant in skin, muscle and liver, whereas trideoxytetrodotoxin preponderated in the ovary. The toxicity of the various tissues was determined by a mouse bioassay.
PMID:17899030 Deiner M et al; Anal Bioanal Chem 389 (6): 1997-2002 (2007)
To investigate the relationship between the toxicity of puffer fish and the distribution of tetrodotoxin-producing bacteria in puffer fish Fugu rubripes collected from the Bohai Sea of China, bacteria were isolated from each organ (ovaries, livers, intestines and gallbladders) and screened for tetrodotoxin (TTX) production. 20 out of 36 isolated strains were found to produce TTX in vitro. In the organs of ovaries and livers whose toxicity is more potent than other organs, the number and toxicity of TTX-producing strains was greater than that of others. Most TTX-producing bacterial strains were identified as Bacillus spp. (19 strains) and Actinomycete spp. (1 strain) based on the morphological observation, physiological and biochemical characteristics and G+C content of DNA. The purified toxin was identified to be TTX by high performance liquid chromatography assay, thin-layer chromatography assay and electrospray ionization mass spectrometry analysis. Our results suggested that TTX-producing bacteria are closely related to the toxification of the puffer fish. More research is needed to elucidate the mechanism of TTX synthesis and the role of TTX in bacteria.
PMID:16051296 Wu Z et al; Toxicon 46 (4): 471-6 (2005)
The liver homogenate of puffer fish was fractionated into blood cell, nuclear, mitochondrial, microsomal and cytosol fractions by the differential centrifugation method. ... Analyses by HPLC and LC-FABMS demonstrated that tetrodotoxin is the major toxic principle in each fraction. These results reveal that tetrodotoxin is widely distributed in organelles in liver cells, though predominantly in the cytosol fraction.
PMID:10519659 Nagashima Y et al; Toxicon 37 (12): 1833-7 (1999)
For more Absorption, Distribution and Excretion (Complete) data for Tetrodotoxin (9 total), please visit the HSDB record page.
The metabolic source of tetrodotoxin is uncertain. No algal source has been identified, and until recently tetrodotoxin was assumed to be a metabolic product of the host. However, recent reports of the production of tetrodotoxin/anhydrotetrodotoxin by several bacterial species, including strains of the family Vibrionaceae, Pseudomonas sp., and Photobacterium phosphoreum, point toward a bacterial origin of this family of toxins.
U.S. FDA, Center for Food Safety and Applied Nutrition. Tetrodotoxin, Foodborne Pathogenic Microorganisms and Natural Toxins Handbook.
To investigate the genes related to the biosynthesis or accumulation of tetrodotoxin (TTX) in pufferfish, mRNA expression patterns in the liver from pufferfish, akamefugu Takifugu chrysops and kusafugu Takifugu niphobles, were compared by mRNA arbitrarily primed reverse transcription-polymerase chain reaction (RAP RT-PCR) with fish bearing different concentrations of TTX and its derivatives. RAP RT-PCR provided a 383 bp cDNA fragment and its transcripts were higher in toxic than non-toxic pufferfish liver. Its deduced amino acid sequence was similar to those of fibrinogen-like proteins reported for other vertebrates. Northern blot analysis and rapid amplification of cDNA ends (RACE) revealed that the cDNA fragment of 383 bp was composed of at least three fibrinogen-like protein (flp) genes, flp-1, flp-2 and flp-3. Relative mRNA levels of flp-1, flp-2 and flp-3 showed a linear correlation with toxicity of the liver for two pufferfish species.
PMID:17360014 Lee JH et al; Toxicon 49 (7): 939-53 (2007)
Sodium current (I(Na)) of the mammalian heart is resistant to tetrodotoxin (TTX) due to low TTX affinity of the cardiac sodium channel (Na(v)) isoform Na(v)1.5. To test applicability of this finding to other vertebrates, TTX sensitivity of the fish cardiac I(Na) and its molecular identity were examined. METHODS: Molecular cloning and whole-cell patch-clamp were used to examine alpha-subunit composition and TTX inhibition of the rainbow trout (Oncorhynchus mykiss) cardiac Na(v) respectively. ...: I(Na) of the trout heart is about 1000 times more sensitive to TTX (IC50 = 1.8-2 nm) than the mammalian cardiac I(Na) and it is produced by three Na(v)alpha-subunits which are orthologs to mammalian skeletal muscle Na(v)1.4, cardiac Na(v)1.5 and peripheral nervous system Na(v)1.6 isoforms respectively. Oncorhynchus mykiss (om) omNa(v)1.4a is the predominant isoform of the trout heart accounting for over 80% of the Na(v) transcripts, while omNa(v)1.5a forms about 18% and omNa(v)1.6a only 0.1% of the transcripts. OmNa(v)1.4a and omNa(v)1.6a have aromatic amino acids, phenylalanine and tyrosine, respectively, in the critical position 401 of the TTX binding site of the domain I, which confers their high TTX sensitivity. More surprisingly, omNa(v)1.5a also has an aromatic tyrosine in this position, instead of the cysteine of the mammalian TTX-resistant Na(v)1.5. CONCLUSIONS: The ortholog of the mammalian skeletal muscle isoform, omNa(v)1.4a, is the predominant Na(v)alpha-subunit in the trout heart, and all trout cardiac isoforms have an aromatic residue in position 401 rendering the fish cardiac I(Na) highly sensitive to TTX.
PMID:17935523 Haverinen J et al; Acta Physiol (Oxf) 191 (3): 197-204 (2007)
... TTX inhibits voltage-gated sodium channels in a highly potent and selective manner without effects on any other receptor and ion channel systems. TTX blocks the sodium channel only from outside of the nerve membrane, and is due to binding to the selectivity filter resulting in prevention of sodium ion flow. It does not impair the channel gating mechanism. More recently, the TTX-resistant sodium channels have been discovered in the nervous system and received much attention because of their role in pain sensation. TTX is now known to be produced not by puffer but by bacteria, and reaches various species of animals via food chain.
PMID:18941294 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2858367 Narahashi T; Proc Jpn Acad Ser B Phys Biol Sci 84 (5): 147-54 (2008)
ABOUT THIS PAGE
74
PharmaCompass offers a list of Tetrodotoxin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Tetrodotoxin manufacturer or Tetrodotoxin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Tetrodotoxin manufacturer or Tetrodotoxin supplier.
PharmaCompass also assists you with knowing the Tetrodotoxin API Price utilized in the formulation of products. Tetrodotoxin API Price is not always fixed or binding as the Tetrodotoxin Price is obtained through a variety of data sources. The Tetrodotoxin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tetrodotoxin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tetrodotoxin, including repackagers and relabelers. The FDA regulates Tetrodotoxin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tetrodotoxin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Tetrodotoxin supplier is an individual or a company that provides Tetrodotoxin active pharmaceutical ingredient (API) or Tetrodotoxin finished formulations upon request. The Tetrodotoxin suppliers may include Tetrodotoxin API manufacturers, exporters, distributors and traders.
click here to find a list of Tetrodotoxin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Tetrodotoxin DMF (Drug Master File) is a document detailing the whole manufacturing process of Tetrodotoxin active pharmaceutical ingredient (API) in detail. Different forms of Tetrodotoxin DMFs exist exist since differing nations have different regulations, such as Tetrodotoxin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Tetrodotoxin DMF submitted to regulatory agencies in the US is known as a USDMF. Tetrodotoxin USDMF includes data on Tetrodotoxin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Tetrodotoxin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Tetrodotoxin suppliers with USDMF on PharmaCompass.
Tetrodotoxin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tetrodotoxin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tetrodotoxin GMP manufacturer or Tetrodotoxin GMP API supplier for your needs.
A Tetrodotoxin CoA (Certificate of Analysis) is a formal document that attests to Tetrodotoxin's compliance with Tetrodotoxin specifications and serves as a tool for batch-level quality control.
Tetrodotoxin CoA mostly includes findings from lab analyses of a specific batch. For each Tetrodotoxin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tetrodotoxin may be tested according to a variety of international standards, such as European Pharmacopoeia (Tetrodotoxin EP), Tetrodotoxin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tetrodotoxin USP).
