
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
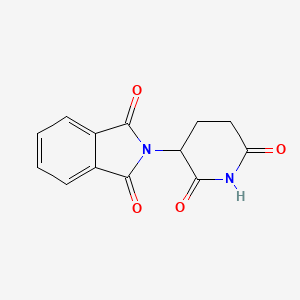

1. Sedoval
2. Thalomid
1. 50-35-1
2. Thalomid
3. 2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione
4. (+/-)-thalidomide
5. Contergan
6. Distaval
7. Pantosediv
8. Softenon
9. Sedoval
10. Kevadon
11. Corronarobetin
12. Psycholiquid
13. Psychotablets
14. Theophilcholine
15. Algosediv
16. Asmadion
17. Bonbrain
18. Calmorex
19. Ectiluran
20. Enterosediv
21. Gastrinide
22. Glutanon
23. Hippuzon
24. Neosedyn
25. Neosydyn
26. Nerosedyn
27. Neufatin
28. Neurodyn
29. Neurosedin
30. Neurosedym
31. Nevrodyn
32. Noctosediv
33. Polygripan
34. Profarmil
35. Quetimid
36. Quietoplex
37. Sandormin
38. Sedimide
39. Sedisperil
40. Shinnibrol
41. Softenil
42. Talargan
43. Talismol
44. Telargean
45. Tensival
46. Thalinette
47. Valgraine
48. Asmaval
49. Calmore
50. Glupan
51. Grippex
52. Imidene
53. Isomin
54. Nibrol
55. Noxodyn
56. Pangul
57. Sleepan
58. Slipro
59. Talimol
60. Telagan
61. Thalin
62. Valgis
63. Yodomin
64. N-phthaloylglutamimide
65. Sedin
66. Predni-sediv
67. Imida-lab
68. N-phthalylglutamic Acid Imide
69. Poly-giron
70. Sedalis Sedi-lab
71. Shin-naito S
72. Neaufatin
73. Asidon 3
74. Pro-ban M
75. 3-phthalimidoglutarimide
76. Imidan (peyta)
77. Neurosedyn
78. Ulcerfen
79. Alpha-phthalimidoglutarimide
80. K-17
81. Bonbrrin
82. Distaxal
83. Distoval
84. Talidomida
85. Kedavon
86. Thaled
87. (+-)-thalidomide
88. 2,6-dioxo-3-phthalimidopiperidine
89. Nsc-66847
90. Thalidomide Celgene
91. Alpha-n-phthalylglutaramide
92. Celgene
93. Pharmion
94. N-(2,6-dioxo-3-piperidyl)phthalimide
95. Glutarimide, 2-phthalimido-
96. N-phthalyl-glutaminsaeure-imid
97. Alpha-(n-phthalimido)glutarimide
98. K 17
99. .alpha.-phthalimidoglutarimide
100. E-217
101. Myrin
102. 1,3-dioxo-2-(2,6-dioxopiperidin-3-yl)isoindoline
103. .alpha.-n-phthalylglutaramide
104. 2-(2,6-dioxopiperidin-3-yl)-1h-isoindole-1,3(2h)-dione
105. 1h-isoindole-1,3(2h)-dione, 2-(2,6-dioxo-3-piperidinyl)-
106. Phthalimide, N-(2,6-dioxo-3-piperidyl)-
107. .alpha.-(n-phthalimido)glutarimide
108. 2-(2,6-dioxo-3-piperidinyl)-1h-isoindole-1,3(2h)-dione
109. Mfcd00153873
110. Chembl468
111. Thalidomide (soluble Form)
112. Nsc-527179
113. 2-(2,6-dioxopiperidin-3-yl)isoindole-1,3-dione
114. 4z8r6ors6l
115. Enmd 0995
116. Thalomide
117. (+-)-n-(2,6-dioxo-3-piperidyl)phthalimide
118. Chebi:74947
119. 2-(2,6-dioxopiperidin-3-yl)-2,3-dihydro-1h-isoindole-1,3-dione
120. Nsc66847
121. Pro-bam M
122. N-(2,6-dioxo-3-piperidinyl)phthalimide
123. Ncgc00015989-09
124. Talidomide [dcit]
125. Thalidomidum
126. Sedalis
127. Talidomide
128. Telargan
129. Dsstox_cid_2524
130. (+/-)-n-(2,6-dioxo-3-piperidyl)phthalimide
131. Dsstox_rid_76611
132. Dsstox_gsid_22524
133. Talidomida [inn-spanish]
134. Thalidomidum [inn-latin]
135. Thalidomine Usp26
136. 2-(2,6-dioxo-piperidin-3-yl)-isoindole-1,3-dione
137. Synovir
138. Talizer
139. (?)-thalidomide
140. Phthalimide,6-dioxo-3-piperidyl)-
141. Wln: T56 Bvnvj C- Dt6vmvtj
142. Thalomid (tm)
143. Thalomid (tn)
144. Thalidomide Pharmion
145. Thaled (tn)
146. N-phthalyl-glutaminsaeure-imid [german]
147. N-phthalimidoglutamic Acid Imide
148. Hsdb 3586
149. 1h-isoindole-1, 2-(2,6-dioxo-3-piperidinyl)-
150. Sr-01000076184
151. Einecs 200-031-1
152. Unii-4z8r6ors6l
153. Nsc 527179
154. Brn 0030233
155. Talinol
156. Thalidomide (jan/usp/inn)
157. Ai3-50606
158. Ccris 8148
159. Nsc-91729
160. Nsc-91730
161. (y)-thalidomide
162. Thalidomide,(s)
163. Cas-50-35-1
164. Prestwick_463
165. Thalidomide [usan:usp:inn:ban:jan]
166. (a+/-)-thalidomide
167. Thalidomide [mi]
168. (+/-)-2-(2,6-dioxo-3-piperidinyl)-1h-isoindole-1,3(2h)-dione
169. Prestwick0_000192
170. Prestwick1_000192
171. Prestwick2_000192
172. Prestwick3_000192
173. Spectrum2_000707
174. Spectrum3_001715
175. Spectrum4_001087
176. Spectrum5_001791
177. Thalidomide [inn]
178. Thalidomide [jan]
179. (.+/-.)-thalidomide
180. Thalidomide [hsdb]
181. Thalidomide [usan]
182. Upcmld-dp139
183. Thalidomide [vandf]
184. Thalomid (tn) (celgene)
185. Schembl7581
186. Nciopen2_003188
187. Thalidomide [mart.]
188. Lopac0_001224
189. Bspbio_000143
190. Bspbio_001156
191. Bspbio_003330
192. Kbiogr_000496
193. Kbiogr_001474
194. Kbiogr_002322
195. Kbioss_000496
196. Kbioss_002324
197. Thalidomide [usp-rs]
198. Thalidomide [who-dd]
199. 2-(2,6-dioxo-3-piperidyl)isoindoline-1,3-dione
200. Mls000069353
201. Divk1c_000051
202. Spectrum1503607
203. Spbio_000893
204. Spbio_002064
205. Thalidomide [ema Epar]
206. Bpbio1_000159
207. Gtpl7327
208. Dtxsid9022524
209. Schembl15197560
210. Upcmld-dp139:001
211. Hms500c13
212. Kbio1_000051
213. Kbio2_000496
214. Kbio2_002322
215. Kbio2_003064
216. Kbio2_004890
217. Kbio2_005632
218. Kbio2_007458
219. Kbio3_000911
220. Kbio3_000912
221. Kbio3_002550
222. Kbio3_002802
223. Thalidomide [orange Book]
224. Cmap_000022
225. Ninds_000051
226. Bio1_000387
227. Bio1_000876
228. Bio1_001365
229. Bio2_000418
230. Bio2_000898
231. Hms1362j17
232. Hms1568h05
233. Hms1792j17
234. Hms1922e12
235. Hms1990j17
236. Hms2090o05
237. Hms2093g15
238. Hms2095h05
239. Hms2234c07
240. Hms3259c22
241. Hms3263f10
242. Hms3266f13
243. Hms3373e06
244. Hms3373g15
245. Hms3403j17
246. Hms3414f19
247. Hms3654a20
248. Hms3678f19
249. Hms3712h05
250. Hms3884i05
251. Pharmakon1600-01503607
252. Thalidomide [usp Monograph]
253. Bcp19772
254. Nsc91729
255. Nsc91730
256. Tox21_110275
257. Tox21_300580
258. Tox21_501224
259. 1h-isoindole-1,3(2h)-dione, 2-(2,6-dioxo-3-piperidinyl)-, (+-)-
260. Ac-917
261. Bbl023439
262. Bdbm50070114
263. Ccg-39878
264. Nsc527179
265. Nsc758479
266. Stl356025
267. ( Inverted Question Mark)-thalidomide
268. Akos009529198
269. Tox21_110275_1
270. Cs-1084
271. Db01041
272. Lp01224
273. Nc00600
274. Nsc-758479
275. Sdccgsbi-0051191.p004
276. (+/-)-thalidomide, >=98%, Powder
277. Idi1_000051
278. Idi1_002173
279. Ncgc00015989-03
280. Ncgc00015989-04
281. Ncgc00015989-05
282. Ncgc00015989-06
283. Ncgc00015989-07
284. Ncgc00015989-08
285. Ncgc00015989-10
286. Ncgc00015989-11
287. Ncgc00015989-12
288. Ncgc00015989-13
289. Ncgc00015989-14
290. Ncgc00015989-16
291. Ncgc00015989-17
292. Ncgc00015989-29
293. Ncgc00024708-02
294. Ncgc00024708-03
295. Ncgc00024708-04
296. Ncgc00024708-05
297. Ncgc00024708-06
298. Ncgc00024708-07
299. Ncgc00024708-08
300. Ncgc00024708-09
301. Ncgc00024708-10
302. Ncgc00024708-11
303. Ncgc00254343-01
304. Ncgc00261909-01
305. 1012310-87-0
306. As-12367
307. Bp-30256
308. Bt164465
309. Hy-14658
310. Nci60_023904
311. Smr000058524
312. Sy052614
313. Wln: T56 Bvnvj C- Dt6vmvtj -d
314. Wln: T56 Bvnvj C- Dt6vmvtj -l
315. Sbi-0051191.p003
316. Db-051759
317. Phthalimide,6-dioxo-3-piperidyl)-, (+)-
318. Phthalimide,6-dioxo-3-piperidyl)-, (-)-
319. Ab00052362
320. Eu-0101224
321. Ft-0600001
322. Ft-0602275
323. Ft-0631211
324. Ft-0675130
325. S1193
326. Sw196678-4
327. T2524
328. Isopropyl (3,4-dichlorophenyl)carbamodithioate
329. Phthalimide,6-dioxo-3-piperidyl)-, D-(+)-
330. Phthalimide,6-dioxo-3-piperidyl)-, L-(-)-
331. C07910
332. D00754
333. Ab00052362-11
334. Ab00052362-12
335. Ab00052362-13
336. Ab00052362_14
337. Ab00052362_15
338. 153t873
339. Q203174
340. Sr-01000076184-1
341. Sr-01000076184-3
342. Sr-01000076184-5
343. Sr-01000076184-8
344. Thalidomide N-(2,6-dioxopiperidin-3-yl)phthalimide
345. W-105969
346. Brd-a93255169-001-04-4
347. Brd-a93255169-001-06-9
348. Brd-a93255169-001-24-2
349. Z1550675451
350. 1h-isoindole-1, 2-(2,6-dioxo-3-piperidinyl)-, (r)-
351. 1h-isoindole-1, 2-(2,6-dioxo-3-piperidinyl)-, (s)-
352. 2-(2,6-dioxo-3-piperidinyl)-1h-isoindole-1,3(2h)-dione #
353. [(r,s)-2-(2,6-dioxo-3-piperidinyl)-1h-isoindole-1,3(2h)-dione
354. Thalidomide, United States Pharmacopeia (usp) Reference Standard
355. ( Inverted Question Mark)-2-(2,6-dioxo-3-piperidinyl)-1h-isoindole-1,3(2h)-dione
356. 1h-isoindole-1,3(2h)-dione, 2-(2,6-dioxo-3-piperidinyl)-, (+/-)-
357. 2-(6-hydroxy-2-oxo-2,3,4,5-tetrahydropyridin-3-yl)-2,3-dihydro-1h-isoindole-1,3-dione
| Molecular Weight | 258.23 g/mol |
|---|---|
| Molecular Formula | C13H10N2O4 |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 258.06405680 g/mol |
| Monoisotopic Mass | 258.06405680 g/mol |
| Topological Polar Surface Area | 83.6 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 449 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Thalomid |
| PubMed Health | Thalidomide (By mouth) |
| Drug Classes | Antineoplastic Agent, Leprostatic |
| Drug Label | THALOMID, -(N-phthalimido) glutarimide, is an immunomodulatory agent. The empirical formula for thalidomide is C13H10N2O4 and the gram molecular weight is 258.2. The CAS number of thalidomide is 50-35-1.... |
| Active Ingredient | Thalidomide |
| Dosage Form | Capsule |
| Route | oral; Oral |
| Strength | 200mg; 150mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Celgene |
| 2 of 2 | |
|---|---|
| Drug Name | Thalomid |
| PubMed Health | Thalidomide (By mouth) |
| Drug Classes | Antineoplastic Agent, Leprostatic |
| Drug Label | THALOMID, -(N-phthalimido) glutarimide, is an immunomodulatory agent. The empirical formula for thalidomide is C13H10N2O4 and the gram molecular weight is 258.2. The CAS number of thalidomide is 50-35-1.... |
| Active Ingredient | Thalidomide |
| Dosage Form | Capsule |
| Route | oral; Oral |
| Strength | 200mg; 150mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Celgene |
Angiogenesis Inhibitors; Immunosuppressive Agents; Leprostatic Agents; Teratogens
National Library of Medicine's Medical Subject Headings. Thalidomide. Online file (MeSH, 2014). Available from, as of December 18, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Thalomid in combination with dexamethasone is indicated for the treatment of patients with newly diagnosed multiple myeloma (MM). /Included in US product label/
NIH; DailyMed. Current Medication Information for Thalomid (Thalidomide) Capsule (Updated: June 2014). Available from, as of February 9, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2eda833b-1357-4ed4-a093-194524fcb061
Thalomid is indicated for the acute treatment of the cutaneous manifestations of moderate to severe erythema nodosum leprosum (ENL). /Included in US product label/
NIH; DailyMed. Current Medication Information for Thalomid (Thalidomide) Capsule (Updated: June 2014). Available from, as of February 9, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2eda833b-1357-4ed4-a093-194524fcb061
Thalomid is also indicated as maintenance therapy for prevention and suppression of the cutaneous manifestations of erythema nodosum leprosum (ENL) recurrence. /Included in US product label/
NIH; DailyMed. Current Medication Information for Thalomid (Thalidomide) Capsule (Updated: June 2014). Available from, as of February 9, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2eda833b-1357-4ed4-a093-194524fcb061
For more Therapeutic Uses (Complete) data for THALIDOMIDE (17 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: EMBRYO-FETAL TOXICITY. If thalidomide is taken during pregnancy, it can cause severe birth defects or embryo-fetal death. Thalidomide should never be used by females who are pregnant or who could become pregnant while taking the drug. Even a single dose (1 capsule (regardless of strength)) taken by a pregnant woman during her pregnancy can cause severe birth defects. Because of this toxicity and in an effort to make the chance of embryo-fetal exposure to Thalomid (thalidomide) as negligible as possible, Thalomid (thalidomide) is approved for marketing only through a special restricted distribution program: Thalomid REMS program, approved by the Food and Drug Administration. This program was formerly known as the "System for Thalidomide Education and Prescribing Safety (S.T.E.P.S. program)".
NIH; DailyMed. Current Medication Information for Thalomid (Thalidomide) Capsule (Updated: June 2014). Available from, as of February 9, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2eda833b-1357-4ed4-a093-194524fcb061
/BOXED WARNING/ WARNING: VENOUS THROMBOEMBOLISM. The use of Thalomid (thalidomide) in multiple myeloma results in an increased risk of venous thromboembolism, such as deep venous thrombosis and pulmonary embolism. This risk increases significantly when thalidomide is used in combination with standard chemotherapeutic agents including dexamethasone. In one controlled trial, the rate of venous thromboembolism was 22.5% in patients receiving thalidomide in combination with dexamethasone compared to 4.9% in patients receiving dexamethasone alone (p = 0.002). Patients and physicians are advised to be observant for the signs and symptoms of thromboembolism. Instruct patients to seek medical care if they develop symptoms such as shortness of breath, chest pain, or arm or leg swelling. Consider thromboprophylaxis based on an assessment of individual patients' underlying risk factors.
NIH; DailyMed. Current Medication Information for Thalomid (Thalidomide) Capsule (Updated: June 2014). Available from, as of February 9, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2eda833b-1357-4ed4-a093-194524fcb061
Use of thalidomide in patients with multiple myeloma is associated with increased risk of venous thromboembolic events (e.g., deep venous thrombosis, pulmonary embolus). Such risk increases substantially when thalidomide is used in combination with standard chemotherapy, including dexamethasone. In a controlled clinical trial, an increased incidence of venous thromboembolic events was observed in patients receiving thalidomide in combination with dexamethasone compared with those receiving dexamethasone alone (22.5 versus 4.9%). Patients and clinicians are advised to watch for signs and symptoms of thromboembolism. Patients should be instructed to notify a clinician if they develop shortness of breath, chest pain, and/or arm or leg swelling.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 3646
Thalidomide is known to cause nerve damage that may be permanent. Peripheral neuropathy is a common (> or =10%) and potentially severe adverse reaction of treatment with thalidomide that may be irreversible. Peripheral neuropathy generally occurs following chronic use over a period of months; however, peripheral neuropathy following relatively short-term use has been reported. The correlation with cumulative dose is unclear. Symptoms may occur some time after thalidomide treatment has been stopped and may resolve slowly or not at all.
NIH; DailyMed. Current Medication Information for Thalomid (Thalidomide) Capsule (Updated: June 2014). Available from, as of February 9, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2eda833b-1357-4ed4-a093-194524fcb061
For more Drug Warnings (Complete) data for THALIDOMIDE (36 total), please visit the HSDB record page.
For the acute treatment of the cutaneous manifestations of moderate to severe erythema nodosum leprosum (ENL). Also for use as maintenance therapy for prevention and suppression of the cutaneous manifestations of ENL recurrence.
FDA Label
Thalidomide BMS in combination with melphalan and prednisone as first line treatment of patients with untreated multiple myeloma, aged > /= 65 years or ineligible for high dose chemotherapy.
Thalidomide BMS is prescribed and dispensed according to the Thalidomide Celgene Pregnancy Prevention Programme (see section 4. 4).
Thalidomide Lipomed in combination with melphalan and prednisone is indicated as first line treatment of patients with untreated multiple myeloma, aged 65 years or ineligible for high dose chemotherapy.
Thalidomide Lipomed is prescribed and dispensed in accordance with the Thalidomide Lipomed Pregnancy Prevention Programme (see section 4. 4).
Thalidomide is an immunomodulatory agent with a spectrum of activity that is not fully characterized. Thalidomide is racemic — it contains both left and right handed isomers in equal amounts: one enantiomer is effective against morning sickness, and the other is teratogenic. The enantiomers are converted to each other in vivo. That is, if a human is given D-thalidomide or L-thalidomide, both isomers can be found in the serum. Hence, administering only one enantiomer will not prevent the teratogenic effect in humans.
Teratogens
An agent that causes the production of physical defects in the developing embryo. (See all compounds classified as Teratogens.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
Leprostatic Agents
Substances that suppress Mycobacterium leprae, ameliorate the clinical manifestations of leprosy, and/or reduce the incidence and severity of leprous reactions. (See all compounds classified as Leprostatic Agents.)
Angiogenesis Inhibitors
Agents and endogenous substances that antagonize or inhibit the development of new blood vessels. (See all compounds classified as Angiogenesis Inhibitors.)
L04AX02
L04AX02
L04AX02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AX - Other immunosuppressants
L04AX02 - Thalidomide
Absorption
The absolute bioavailability has not yet been characterized in human subjects due to its poor aqueous solubility. In studies of both healthy volunteers and subjects with Hansen’s disease, the mean time to peak plasma concentrations (Tmax) ranged from 2.9 to 5.7 hours indicating that thalidomide is slowly absorbed from the gastrointestinal tract.
Route of Elimination
Thalidomide itself has less than 0.7% of the dose excreted in the urine as unchanged drug.
... Thalidomide given orally to rats was poorly absorbed.
Hayes, W.J., Jr., E.R. Laws Jr., (eds.). Handbook of Pesticide Toxicology Volume 1. General Principles. New York, NY: Academic Press, Inc., 1991., p. 76
In animal studies, high concentrations of thalidomide were found in the gastrointestinal tract, liver, and kidney; and lower concentrations were found in the muscle, brain, and adipose tissue. Thalidomide crosses the placenta. It is not known whether thalidomide is present in the ejaculate of males.
USP. Convention. USPDI - Drug Information for the Health Care Professional. 19th ed. Volume I.Micromedex, Inc. Englewood, CO., 1999. Content Prepared by the U.S. Pharmacopieal Convention, Inc., p. 2778
Thalidomide has a renal clearance of 1.15 mL per minute; less than 0.7% of the total dose is excreted unchanged.
USP. Convention. USPDI - Drug Information for the Health Care Professional. 19th ed. Volume I.Micromedex, Inc. Englewood, CO., 1999. Content Prepared by the U.S. Pharmacopieal Convention, Inc., p. 2778
... The present study determined the bioequivalence and pharmacokinetics of ... commercial and clinical trial thalidomide formulations and the Brazillian Tortuga formulation in an open label, single dose, three-way crossover design. ... The terminal rate constant for the Tortuga formulation was significantly less, giving rise to a terminal half-life of 15 hr compared to about 5-6 hr in the /Commercial/ formulations. ... Extent of absorption, as measured by AUC0-infinity was approx equal for all three formulations. Terminal half-life for Tortuga was two to three times longer than compared to the /commercial/ formulations and is clear evidence for absorption rate limitations. The two ... /commercial/ formulations showed similar pharmacokinetic parameters with profiles that were best described by one compartment model with first order absorption and elimination. ...
Teo SK, et al; J Clin Pharm 39 (11): 1162-8 (1999)
For more Absorption, Distribution and Excretion (Complete) data for THALIDOMIDE (20 total), please visit the HSDB record page.
Thalidomide itself does not appear to be hepatically metabolized to any large extent, but appears to undergo non-enzymatic hydrolysis in plasma to multiple metabolites. Thalidomide may be metabolized hepatically by enzymes of the cytochrome P450 enzyme system. The end product of metabolism, phthalic acid, is excreted as a glycine conjugate.
Studies on thalidomide metabolism in humans have not been done. In animals, nonenzymatic hydrolytic cleavage appears to be the main pathway of degradation, producing seven major and at least five minor hydrolysis products. Thalidomide may be metabolized hepatically by the enzymes of the cytochrome p450 enzyme system. Thalidomide does not appear to induce or inhibit its own metabolism. However, it may interfere with enzyme induction caused by other compounds. The end product of metabolism, phthalic acid, is excreted as a glycine conjugate.
USP. Convention. USPDI - Drug Information for the Health Care Professional. 19th ed. Volume I.Micromedex, Inc. Englewood, CO., 1999. Content Prepared by the U.S. Pharmacopieal Convention, Inc., p. 2778
The chiral inversion and hydrolysis of thalidomide and the catalysis by bases and human serum albumin were investigated by /utilizing/ a stereoselective HPLC assay. Chiral inversion was catalyzed by albumin, hydroxyl ions, phosphate and amino acids. Basic amino acids (arginine and lysine) had a superior potency in catalyzing chiral inversion compared to acid and neutral ones. The chiral inversion of thalidomide is thus subject to specific and general base catalysis and it is suggested that the ability of HSA to catalyze the reaction is due to basic groups of the amino acids arginine and lysine and not to a single catalytic site on the macromolecule. The hydrolysis of thalidomide was also base catalyzed. ... Albumin had no effect on hydrolysis and there was no difference between the catalytic potencies of acidic, neutral and base amino acids. ... Chiral inversion is deduced to occur by electrophilic substitution involving specific and general base catalysis, whereas hydrolysis is thought to occur by nucleophilic substitution involving specific and general base as well as nucleophilic catalysis. As nucleophilic attack is sensitive to steric properties of the catalyst, steric hindrance might be the reason albumin is not able to catalyze hydrolysis. (1)H NMR experiments revealed that the three teratogenic metabolites of thalidomide, in sharp contrast to the drug itself had complete chiral stability. This leads to the speculation that, were some enantioselectivity to exist in the teratogenicity of thalidomide, it could result from fast hydrolysis to chirally stable teratogenic metabolites.
PMID:9860497 Reist M, et al; Chem Res Toxicol 11 (12): 1521-8 (1998)
Thalidomide has been shown to be an inhibitor of angiogenesis in a rabbit cornea micropocket model; however, it has failed to demonstrate this activity in other models. These results suggest that the anti-angiogenic effects of thalidomide may only be observed following metabolic activation of the compound. This activation process may be species specific, similar to the teratogenic properties associated with thalidomide. Using a rat aorta model and human aortic endothelial cells, we co-incubated thalidomide in the presence of either human, rabbit, or rat liver microsomes. These experiments demonstrated that thalidomide inhibited microvessel formation from rat aortas and slowed human aortic endothelial cell proliferation in the presence of human or rabbit microsomes, but not in the presence of rat microsomes. In the absence of microsomes, thalidomide had no effect on either microvessel formation or cell proliferation, thus demonstrating that a metabolite of thalidomide is responsible for its anti-angiogenic effects and that this metabolite can be formed in both humans and rabbits, but not in rodents. /There are five primary metabolites of thalidomide [4-OH-thalidomide, 3-OH-thalidomide, 39-OH-thalidomide, 49-OH-thalidomide, and 59-OH-thalidomide], and the antiangiogenic property could be the result of either of these compounds, or of an intermediate. Also, thalidomide undergoes rapid spontaneous hydrolysis in aqueous solutions at a pH of 6.0 or greater to form three primary products [4-phthalimidoglutaramic acid, 2-phthalimidoglutaramic acid, and a-(o-carboxybenzamido) glutarimide] and eight minor products. Furthermore, each of the five metabolites of the parent compound undergoes similar hydrolysis./
PMID:9714301 Bauer KS, et al; Biochem Pharmacol 55 (11): 1827-34 (1998)
Three CD-1 mice were dosed orally with 3000 mg/kg thalidomide in 1% carboxymethylcellulose daily for three days and plasma samples were obtained 2, 4 and 6 hours postdose on the third day. Extracts of mouse plasma from thalidomide treated mice contained at least four components that absorbed at 230 nm, not observed in control plasma extracts. The first two components did not match any standards and may represent other metabolites, possibly hydrolysis products of thalidomide. The second pair of components closely matched standards for 4-hydroxythhalidomide and thalidomide respectively.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Thalidomide Pharmion (Thalidomide) p.10 (2008). Available from, as of February 10, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000823/WC500037054.pdf
For more Metabolism/Metabolites (Complete) data for THALIDOMIDE (7 total), please visit the HSDB record page.
The mean half-life of elimination ranges from approximately 5 to 7 hours following a single dose and is not altered upon multiple dosing.
... The pharmacokinetics and hemodynamic effects of two oral doses of thalidomide (100 and 200 mg) were investigated, using a randomized two period crossover design, in a group of asymptomatic male HIV seropositive subjects. Thalidomide pharmacokinetics were linear at the doses studied, and were best described by a one compartment model with first order absorption and elimination processes. The drug was rapidly absorbed with a mean absorption half life of 0.95 hr (range 0.16-2.49 hr) and 1.19 hr (0.33-3.53 hr) after 100 and 200 mg doses, respectively. The corresponding Cmax values were 1.15 +/-0.24 ug/mL (100 mg) and 1.92 +/- 0.47- ug/mL (200 mg; p<0.001) which were achieved (Tmax) at 2.5 +/-1.5 hr and 3.3 +/-1.4 hr, respectively. Plasma concn of thalidomide declined thereafter, in a log linear manner, with elimination half lives of 4.6+/-1.2 hr (100 mg) and 5.3+/-2.2 hr -(200 mg). The apparent volumes of distribution (Vdss/F) were 69.9+/-1.56 L (100 mg) and 82.7+/-34.9 L (200 mg) while total body clearances (C1F) were 10.4+/-2.1 and 10.8+/- 1.7 L/hr, respectively. ...
Noormohamed FH, et al; Airs Res Human Retroviruses 15 (12): 1047-52 (1999)
The mean elimination half-life of thalidomide following a single 200-mg oral dose ranges from 3-6.7 hours and the elimination half-life appears to be similar following multiple doses of the drug. In a study in healthy adults who received a single 50-, 200-, or 400-mg oral dose of the drug, the mean elimination half-life of thalidomide was 5.5, 5.5, or 7.3 hours, respectively. The mean elimination half-life of thalidomide was 6.9 hours in adults with leprosy who received a single 400-mg oral dose and 4.6-6.5 hours in HIV-infected adults who received a single 100- to 300-mg dose.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 3653
In patients with erythema nodosum leprosum (ENL) the mechanism of action is not fully understood. Available data from in vitro studies and preliminary clinical trials suggest that the immunologic effects of this compound can vary substantially under different conditions, but may be related to suppression of excessive tumor necrosis factor-alpha (TNF-a) production and down-modulation of selected cell surface adhesion molecules involved in leukocyte migration. For example, administration of thalidomide has been reported to decrease circulating levels of TNF-a in patients with ENL, however, it has also been shown to increase plasma TNF-a levels in HIV-seropositive patients. As a cancer treatment, the drug may act as a VEGF inhibitor.
The sedative drug thalidomide ([+]-alpha-phthalimidoglutarimide), once abandoned for causing birth defects in humans, has found new therapeutic license in leprosy and other diseases, with renewed teratological consequences. Although the mechanism of teratogenesis and determinants of risk remain unclear, related teratogenic xenobiotics are bioactivated by embryonic prostaglandin H synthase (PHS) to a free-radical intermediates that produce reactive oxygen species (ROS), which cause oxidative damage to DNA and other cellular macromolecules. Similarly, thalidomide is bioactivated by horseradish peroxidase, and oxidizes DNA and glutathione, indicating free radical-mediated oxidative stress. Furthermore, thalidomide teratogenicity in rabbits is reduced by the PHS inhibitor acetylsalicylic acid, indicating PHS-catalyzed bioactivation. Here, we show in rabbits that thalidomide initiates embryonic DNA oxidation and teratogenicity, both of which are abolished by pre-treatment with the free radical spin trapping agent alpha-phenyl-N-t-butylnitrone (PBN). In contrast, in mice, a species resistant to thalidomide teratogenicity, thalidomide does not enhance DNA oxidation, even at a dose 300% higher than that used in rabbits, providing insight into an embryonic determinant of species-dependent susceptibility. In addition to their therapeutic implications, these results constitute direct evidence that the teratogenicity of thalidomide may involve free radical-mediated oxidative damage to embryonic cellular macromolecules.
PMID:10229238 Parman T, et al; Nature Medicine 5 (5): 582-5 (1999)
The glutamic acid derivative thalidomide is a transcriptional inhibitor of TNF-alpha but is also known to affect human blood vessels, which may underlie its teratogenicity. Thalidomide has been used in the treatment of refractory Crohn's disease (CD), but the therapeutic mechanism is not defined. We examined the effect of thalidomide on primary cultures of human intestinal microvascular endothelial cells (HIMEC), the relevant endothelial cell population in inflammatory bowel disease (IBD), to determine its effect on endothelial activation, leukocyte interaction, and VEGF-induced angiogenesis. HIMEC cultures were pretreated with thalidomide before activation with either TNF-alpha/LPS or VEGF. A low-shear-stress flow adhesion assay with either U-937 or whole blood was used to assess HIMEC activation following TNF-alpha/LPS, and a Wright's stain identified adherent leukocytes. Expression of cell adhesion molecules (E-selectin, intercellular adhesion molecule-1, vascular cell adhesion molecule-1) was assessed using radioimmunoassay. Effects of thalidomide on NF-kappaB activation, cyclooxygenase (COX)-2, and inducible nitric oxide synthase (iNOS) expression in TNF-alpha/LPS-activated HIMEC were determined by RT-PCR and Western blotting. Thalidomide blocked adhesion of both U-937 and whole blood leukocytes by 50% in HIMEC, inhibiting binding of all classes of leukocytes. Thalidomide also blocked NF-kappaB and cell adhesion molecule expression in HIMEC. In marked contrast, thalidomide did not affect either iNOS or COX-2 expression, two key molecules that play a role in the downregulation of HIMEC activation. VEGF-induced HIMEC transmigration, growth, proliferation, tube formation, and Akt phosphorylation were significantly inhibited by thalidomide. In summary, thalidomide exerted a potent effect on HIMEC growth and activation, suggesting that it may also function via an endothelial mechanism in the treatment of CD.
PMID:19926820 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2822501 Rafiee P et al; Am J Physiol Gastrointest Liver Physiol 298 (2): G167-76 (2010)
Thalidomide has been shown to have species metabolic dependent antiangiogenic activity in vitro and in vivo, suggesting its potential in treating human angiogenesis dependent pathologies such as solid tumors. ... Thalidomide treated LNCaP /prostate/ cells demonstrated an incr prostate specific antigen/cell levels at all concn tested compared to untreated control cells. Thalidomide demonstrated a cytostatic effect in LNCaP cells but had no appreciable effect on PC-3 cell viability compared to untreated control cells. Comparison of cDNA expression arrays hybridized with thalidomide treated LNCaP cDNA probes suggests that thalidomide may up or down regulate expression of angiogenesis related genes, ie. vitronectin, but these differential effects require further verification. Thalidomide over a range of doses has demonstrated nontoxic, cytostatic activity in LNCaP cells and significant up regulation of LNCaP cell prostate specific antigen secretion in vitro. ... Preliminary data from cDNA nucleic acid arrays of thalidomide treated LNCaP cells suggest that thalidomide up regulates a potential angiogenic modulatory protein, the vitronectin precursor, which may eventually link thalidomide's antiangiogenic activity with modulation of antiogenic vascular pathways.
Dixon SC, et al; Cancer Chemother Pharmacol 43: PS78-84 (1999)
Thalidomide was initially used as a sedative during pregnancy but was withdrawn from the market due to its teratogenic effects. In vitro studies have shown that thalidomide inhibits tumour necrosis factor alpha (TNF-alpha) mRNA expression and protein production by mitogen-stimulated macrophages and activated T cells. Even at the highest concentration (10-1 mM) tested, however, TNF-alpha levels are inhibited only partially and the mechanism of action is unknown. In the present investigations, we have examined the influence of thalidomide on nuclear levels of NF-kappa B in human peripheral blood mononuclear cells (PBMC) following activation with mitogen or phorbol myristate acetate (PMA)/ionophore. Dexamethasone was used as a positive control due to its well-characterised mechanism of action and NF-kappa B-mediated effects on TNF-alpha expression. PBMC from healthy human volunteers were stimulated optimally with phytohemagglutinin (PHA) or PMA/ionophore in the presence of 10(-1)-10(-5) mM thalidomide or dexamethasone, concentrations that displayed a range of inhibitory effects on TNF-alpha production. Cells were harvested at varying time points and nuclear extracts prepared. Nuclear levels of NF-kappa B were measured using electrophoretic mobility shift assays (EMSA) with a radiolabelled DNA probe specific for NF-kappa B. Results were analysed using optical densitometry. Nuclear levels of NF-kappa B were found to be unaffected by thalidomide at all concentrations tested, including concentrations (10(-1)-10(-3) mM) that exhibited significant inhibition of TNF-alpha protein and mRNA expression. In concurrent experiments, dexamethasone was found to reduce NF-kappa B expression in a dose-dependent manner with maximal inhibition at the highest dose tested (10(-1) mM). TNF-alpha gene expression is controlled by at least three separate transcription factors that are involved in binding to the promoter region. These observations suggest that thalidomide does not act directly on NF-kappa B and therefore inhibits TNF-alpha production through another independent mechanism.
PMID:11367517 Rowland TL et al; Int Immunopharmacol 1 (1): 49-61 (2001)
For more Mechanism of Action (Complete) data for THALIDOMIDE (15 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
15
PharmaCompass offers a list of Thalidomide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Thalidomide manufacturer or Thalidomide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Thalidomide manufacturer or Thalidomide supplier.
PharmaCompass also assists you with knowing the Thalidomide API Price utilized in the formulation of products. Thalidomide API Price is not always fixed or binding as the Thalidomide Price is obtained through a variety of data sources. The Thalidomide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Thalomid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Thalomid, including repackagers and relabelers. The FDA regulates Thalomid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Thalomid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Thalomid manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Thalomid supplier is an individual or a company that provides Thalomid active pharmaceutical ingredient (API) or Thalomid finished formulations upon request. The Thalomid suppliers may include Thalomid API manufacturers, exporters, distributors and traders.
click here to find a list of Thalomid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Thalomid DMF (Drug Master File) is a document detailing the whole manufacturing process of Thalomid active pharmaceutical ingredient (API) in detail. Different forms of Thalomid DMFs exist exist since differing nations have different regulations, such as Thalomid USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Thalomid DMF submitted to regulatory agencies in the US is known as a USDMF. Thalomid USDMF includes data on Thalomid's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Thalomid USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Thalomid suppliers with USDMF on PharmaCompass.
A Thalomid written confirmation (Thalomid WC) is an official document issued by a regulatory agency to a Thalomid manufacturer, verifying that the manufacturing facility of a Thalomid active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Thalomid APIs or Thalomid finished pharmaceutical products to another nation, regulatory agencies frequently require a Thalomid WC (written confirmation) as part of the regulatory process.
click here to find a list of Thalomid suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Thalomid as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Thalomid API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Thalomid as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Thalomid and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Thalomid NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Thalomid suppliers with NDC on PharmaCompass.
Thalomid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Thalomid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Thalomid GMP manufacturer or Thalomid GMP API supplier for your needs.
A Thalomid CoA (Certificate of Analysis) is a formal document that attests to Thalomid's compliance with Thalomid specifications and serves as a tool for batch-level quality control.
Thalomid CoA mostly includes findings from lab analyses of a specific batch. For each Thalomid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Thalomid may be tested according to a variety of international standards, such as European Pharmacopoeia (Thalomid EP), Thalomid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Thalomid USP).
