
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
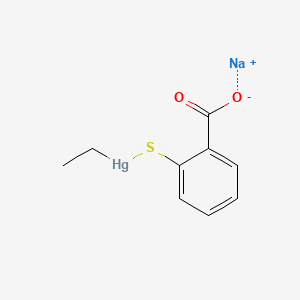

1. Ethylmercurithiosalicylate, Sodium
2. Mercurothiolate
3. Merthiolate
4. Sodium Ethylmercurithiosalicylate
5. Thiomersal
6. Thiomersalate
7. Vitaseptol
1. Thiomersal
2. 54-64-8
3. Thiomersalate
4. Mercurothiolate
5. Merthiolate
6. Sodium Merthiolate
7. Sodium Ethylmercurithiosalicylate
8. Ethylmercurithiosalicylate Sodium
9. Ethylmercurithiosalicylic Acid Sodium Salt
10. Thiomersalum
11. Tiomersal
12. Ethyl(2-mercaptobenzoato-s)mercury Sodium Salt
13. O-(ethylmercurithio)benzoic Acid Sodium Salt
14. Thiomersal [inn]
15. ((o-carboxyphenyl)thio)ethylmercury Sodium Salt
16. Sodium;(2-carboxylatophenyl)sulfanyl-ethylmercury
17. [(o-carboxyphenyl)thio]ethylmercury Sodium Salt
18. 2225pi3mov
19. Chebi:9546
20. Thimerosalate
21. Thimerosalum
22. Thimerosol
23. Thimersalate
24. Thiomersalat
25. Thiomersal (inn)
26. Ncgc00094791-01
27. Elicide
28. Estivin
29. Merfamin
30. Merphol
31. Mertorgan
32. Merzonin
33. Nosemack
34. Tiomersale
35. Vitaseptol
36. Mercurothiolatum
37. Merzonin Sodium
38. Aeroaid Spray
39. Merthiolate Salt
40. Dsstox_cid_5540
41. Merthiolate Sodium
42. Vitaseptol Loesung
43. Dsstox_rid_77823
44. Dsstox_gsid_25540
45. Merseptyl (van)
46. Elcide 73
47. Elcide 75
48. Tiomersale [dcit]
49. Caswell No. 766
50. Mls001336050
51. Sodium Ethylmercuric Thiosalicylate
52. Tiomersal [inn-spanish]
53. Thiomersalum [inn-latin]
54. Sodium O-(ethylmercurithio)benzoate
55. Sodium 2-(ethylmercurithio)benzoate
56. Cas-54-64-8
57. Ethylmercurithiosalicylate Sodium Salt
58. Ethylmerkurithiosalicilan Sodny
59. Ccris 4839
60. Hsdb 7151
61. Thimerosal [usp:jan]
62. Nsc 4794
63. Smr000875330
64. Einecs 200-210-4
65. Mfcd00013062
66. Epa Pesticide Chemical Code 078901
67. Ethylmerkurithiosalicilan Sodny [czech]
68. Ethyl (sodium O-mercaptobenzoato)mercury
69. Unii-2225pi3mov
70. Thime-rosal
71. Ethylmercurithiosalicyclic Acid, Sodium Salt
72. Mercurate(1-), Ethyl(2-mercaptobenzoato(2-)-o,s)-, Sodium
73. Merthiolate (tn)
74. Mercury, Ethyl(2-mercaptobenzoate-s)-, Sodium Salt
75. Mercury, Ethyl(2-mercaptobenzoato-s)-, Sodium Salt
76. Mercury, ((o-carboxyphenyl)thio)ethyl-, Sodium Salt
77. Prestwick_1021
78. Mercurate(1-), Ethyl(o-mercaptobenzoato(2-))-, Sodium
79. Thimerosal [ii]
80. Thimerosal [mi]
81. Thimerosal (jan/usp)
82. Thimerosal [jan]
83. Mercurate(1-), Ethyl(2-mercaptobenzoate(2-)-o,s)-, Sodium Salt
84. Thimerosal [hsdb]
85. Thimerosal [inci]
86. Thimerosal [vandf]
87. Epitope Id:119682
88. Schembl3525
89. Thiomersal [mart.]
90. Ethyl(2-mercaptobenzoato-s)mercury, Sodium Salt
91. Thimerosal [usp-rs]
92. Thiomersal [who-dd]
93. Sodium (2-carboxylatophenyl)sulfanyl-ethyl-mercury
94. Ethylmercury(1+) Sodium 2-sulfidobenzoate(1:1:1)
95. Mls001336049
96. Set
97. Spectrum1500572
98. Mercury, Ethyl (2-mercaptobenzoato-s)-, Sodium Salt
99. Thimerosal, Analytical Standard
100. Dtxsid3025540
101. Thimerosal, >=97% (hplc)
102. Hms501o19
103. Mercury, Ethyl(hydrogen O-mercaptobenzoato)-, Sodium Salt
104. Thimerosal, >=95.0% (hg)
105. Thiomersal [ep Monograph]
106. Bdbm512713
107. Hms1921e07
108. Hms2092m09
109. Hms2230i04
110. Hms3371n19
111. Sodium Ethyl[2-(sulfanyl-kappas)benzoato(2-)]mercurate(1-)
112. Srct-03744
113. Thimerosal [usp Monograph]
114. Thimerosal, Bioxtra, 97-101%
115. Nsc-4794
116. Tox21_111331
117. Tox21_302176
118. Ccg-39725
119. Akos004910446
120. Akos037503710
121. Mercurate(1-), Ethyl(2-(mercapto-kappas)benzoato(2-)-kappao)-, Sodium
122. Tox21_111331_1
123. Db11590
124. Ncgc00094791-02
125. Ncgc00164425-01
126. Ncgc00178879-04
127. Ncgc00255169-01
128. Bp-30041
129. Db-052622
130. Ft-0603231
131. Thimerosal, Meets Usp Testing Specifications
132. Sodium (2-carboxylatophenylthio)(ethyl)mercury
133. D00864
134. H10793
135. Sodium [(2-carboxylatophenyl)thio]-ethylmercury
136. T-3600
137. Ethylmercurithiosalicylic Acid Na Thimerosal
138. A830286
139. Q411046
140. Sodium Ethyl[2-(mercapto-ks)benzoato(2-)]mercurate(1-)
141. Sodium Salt Of (2-carboxyphenylthio)ethylmercury
142. Sodium [(2-carboxylatophenyl)sulfanyl](ethyl)mercurate(1-)
143. Thimerosal, European Pharmacopoeia (ep) Reference Standard
144. Thimerosal, United States Pharmacopeia (usp) Reference Standard
145. Thimerosal Ready Made Solution, 100 Mg/ml In Water, 0.2 Mum Filtered
146. Thimerosal, Pharmaceutical Secondary Standard; Certified Reference Material
147. Mercurate(1-), Ethyl(2-(mercapto-kappas)benzoato(2-)-kappao)-, Sodium (1:1)
148. Sodium Ethyl(2-(mercapto-.kappa.s)benzoato(2-)-.kappa.o)mercurate(1-)
| Molecular Weight | 404.82 g/mol |
|---|---|
| Molecular Formula | C9H9HgNaO2S |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 405.992739 g/mol |
| Monoisotopic Mass | 405.992739 g/mol |
| Topological Polar Surface Area | 65.4 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 180 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
VET: Antibacterial, antifungal (topical)
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1661
... As a precautionary public health effort to minimize exposure of mercury to infants and children, a joint statement was issued in July 1999 by the American Academy of Pediatrics and the U.S. Public Health Service recommending the removal of thimerosal from vaccines as soon as possible. Today, all routinely recommended pediatric vaccines manufactured for the U.S. market contain no thimerosal or only trace amounts. ...
National Academy of Sciences; Institute of Medicine. Available from, as of March 14, 2004: https://www.iom.edu/focuson.asp?id=4189
In June 2000, a joint statement on thimerosal in vaccines was prepared by the American Academy of Family Physicians (AAFP), the American Academy of Pediatrics (AAP), the Advisory Committee on Immunization Practices (ACIP), and the Public Health Service (PHS) in response to 1) the progress in achieving the national goal declared in July 1999 to remove thimerosal from vaccines in the recommended childhood vaccination schedule, and 2) results of recent studies that examined potential associations between exposure to mercury in thimerosal-containing vaccines and health effects. In this statement, AAFP, AAP, ACIP, and PHS recommend continuation of the current policy of moving rapidly to vaccines that are free of thimerosal as a preservative. Until adequate supplies are available, use of vaccines that contain thimerosal as a preservative is acceptable.
Morb Mortal Wkly Rep (MMWR) 49 (27): 622, 631 (2000)
Used as preservative in some cosmetics, topical pharmaceuticals, and biological drug products, which includes vaccines.
Thimerosal is an organomercurial compound and derivative of thiosalicyclic acid with antibacterial and antifungal properties. Thimerosal, which consists of approximately 50% mercury by weight, has been one of the most widely used preservatives in vaccines. It is metabolized/degraded to ethylmercury and thiosalicylate. Ethylmercury is an organomercurial that must be carefully distinguished from methylmercury, a closely related substance that has been the focus of many studies. Methylmercury is the type of mercury found in various species of fish. Experimental data demonstrates that the toxicokinetics of thimerosal (ethylmercury) is vastly different from that of methyl-mercury. Thus, methyl-mercury is not a suitable reference for assessing the risk from exposure to thimerosal-derived mercury. Prior to the recent initiative to reduce or eliminate thimerosal from childhood vaccines, the maximum cumulative exposure to mercury via routine childhood vaccinations during the first 6 months of life was 187.5 micrograms. In the most recently formulated vaccines, the maximum cumulative exposure during the first 6 months of life should now be less than 3 micrograms of mercury. Currently, thimerosal may still be used in the early stages of manufacturing of certain childhood vaccines, however, only a trace remains after a chemical purification process. Note that the dose above is indicated for children 1-6 months of age is applicable only in the United States, and other countries may have varying indications.
Preservatives, Pharmaceutical
Substances added to pharmaceutical preparations to protect them from chemical change or microbial action. They include ANTI-BACTERIAL AGENTS and antioxidants. (See all compounds classified as Preservatives, Pharmaceutical.)
D - Dermatologicals
D08 - Antiseptics and disinfectants
D08A - Antiseptics and disinfectants
D08AK - Mercurial products
D08AK06 - Thiomersal
Absorption
Less than 0.01% of an ingested dose is absorbed from the GI tract (rat study).
Route of Elimination
Gastrointestinal tract.
Volume of Distribution
266 L in one study
Clearance
The high concentrations of mercury identified in stool samples suggest that ethylmercury may be eliminated through the gastrointestinal tract.
Mercury concentrations were measured in the aqueous humor and excised corneal buttons of nine patients undergoing keratoplasty. A contact lens stored for several weeks in a solution containing thimerosal was applied to one eye for 4 hours. After 4 hours the lens was removed and mercury concentrations were determined in aqueous humor, corneal buttons, and the contact lens itself. Markedly elevated levels of mercury were determined in both aqueous humor and corneal buttons of subjects as compared to controls; however, there was little residual mercury on the contact lens after 4 hours.The mercury content in the corneal buttons of subjects ranged from 0.6 to 14 ng per tissue. The mercury content in samples of aqueous humor from subjects ranged from 20 to 46 ng/mL.
Goldfrank, L.R., Flomenbaum, N.E., Lewin, N.A., Weisman, R.S., Howland, M.A., Hoffman, R.S., Goldfrank's Toxicologic Emergencies 6th Ed. (1998)., McGraw-Hill, New York, N.Y., p. 918
Ten of 13 infants exposed to topical applications of a thimerosal tincture 0.1% for the treatment of exomphalos died. The total number of applications ranged from 9 to 48. Mercury concentrations were determined in various tissues from 6 of the infants. Mean tissue concentrations in fresh samples of liver, kidney, spleen, and heart ranged from 5152 to 11,330 ppb, suggesting percutaneous absorption from repeated topical applications.
Goldfrank, L.R., Flomenbaum, N.E., Lewin, N.A., Weisman, R.S., Howland, M.A., Hoffman, R.S., Goldfrank's Toxicologic Emergencies 6th Ed. (1998)., McGraw-Hill, New York, N.Y., p. 918
Urine mercury levels were studied in 26 patients with hypogammaglobulinemia who received intramuscular weekly IgG replacement therapy preserved with 0.01% thimerosal. The dosage of IgG ranged from 25 mg/kg to 50 mg/kg, containing 0.6-1.2 mg of mercury per dose. The total estimated dose of mercury administered ranged from 4 to 734 mg over a period of 6 months to 17 years. Elevated urine mercury levels were determined in 19 patients; however, no patients had clinical evidence of chronic mercury toxicity.
Goldfrank, L.R., Flomenbaum, N.E., Lewin, N.A., Weisman, R.S., Howland, M.A., Hoffman, R.S., Goldfrank's Toxicologic Emergencies 6th Ed. (1998)., McGraw-Hill, New York, N.Y., p. 918
Forty full-term infants aged 6 months and younger were given vaccines that contained thiomersal (diptheria-tetanus-acellular pertussis vaccine, hepatitis B vaccine, and in some children Haemophilus influenzae type b vaccine). 21 control infants received thiomersal-free vaccines. We obtained samples of blood, urine, and stools 3-28 days after vaccination. Total mercury (organic and inorganic) in the samples was measured by cold vapour atomic absorption. Mean mercury doses in infants exposed to thiomersal were 45.6 microg (range 37.5-62.5) for 2-month-olds and 111.3 microg (range 87.5-175.0) for 6-month-olds. Blood mercury in thiomersal-exposed 2-month-olds ranged from less than 3.75 to 20.55 nmol/L (parts per billion); in 6-month-olds all values were lower than 7.50 nmol/L. Only one of 15 blood samples from controls contained quantifiable mercury. Concentrations of mercury were low in urine after vaccination but were high in stools of thiomersal-exposed 2-month-olds (mean 82 ng/g dry weight) and in 6-month-olds (mean 58 ng/g dry weight). Estimated blood half-life of ethylmercury was 7 days (95% CI 4-10 days). Administration of vaccines containing thiomersal does not seem to raise blood concentrations of mercury above safe values in infants. Ethylmercury seems to be eliminated from blood rapidly via the stools after parenteral administration of thiomersal in vaccines.
PMID:12480426 Pichichero ME et al; Lancet 360 (9347): 1737-41(2002)
Ethylmercury (etHg) is derived from the metabolism of thimerosal (o-carboxyphenyl-thio-ethyl-sodium salt), which is the most widely used form of organic mercury.
A study was done to study the pharmacokinetics of Thimerosal in mice. Estimated half-lives (in days) were 8.8 for blood, 10.7 for brain, 7.8 for heart, 7.7 for liver and 45.2 for kidney. The the long half-life of ethylmercury (~50 days on average in humans) results in accumulation that may be harmful to the developing fetal brain, as it is more susceptible to organomercurial compounds than the adult brain.
Although its mechanism of action is not fully understood, thimerosal inhibits sulfhydryl-containing active site of various enzymes and binds to sulfhydryl compounds, including glutathione, cysteine, and sulfhydryl groups of proteins. In addition, thimerosal activates the InsP3 calcium channel on the endoplasmic reticular membrane, thereby triggering the release of intracellular calcium resulting in a calcium-induced calcium-influx of extracellular calcium. Therefore, thimerosal may induce or inhibit various cellular functions that are dependent on the signaling of calcium. Ethylmercury is metabolized to inorganic mercury more rapidly than methylmercury. This difference in metabolism may account for kidney pathology that can result from toxic quantities. Also, whereas the increase in oxidative stress and induction of apoptosis observed in vitro with large doses (405 g/L to 101 mg/L) of thimerosal may explain its damaging neurological effects. The effects of low-dose ethylmercury are not completely understood to date. It is known, however, that the shorter half-life of ethylmercury (the metabolite of thimerosal) allows for very limited opportunities of ethylmercury derived from thimerosal in vaccines. Ethylmercury is a lipophilic cation that is capable of crossing the blood-brain barrier. The octanol/water partition coefficients of methyl and ethylmercury are 1.4 to 1.8, at intracellular pH and [Cl], therefore, both organomercury compounds will primarily exist as intracellular lipophilic cations. It has been demonstrated that lipophilic cations accumulate inside mitochondria, in a Nernstian fashion, driven by the steady state membrane potential. As the typical mitochondrial membrane potential of astrocytes and neurons is between 140170mV, one would expect the concentration of these organomercury compounds within mitochondria to be approximately 1000 times greater than the cytosolic concentration.
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
29
PharmaCompass offers a list of Thimerosal API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Thimerosal manufacturer or Thimerosal supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Thimerosal manufacturer or Thimerosal supplier.
PharmaCompass also assists you with knowing the Thimerosal API Price utilized in the formulation of products. Thimerosal API Price is not always fixed or binding as the Thimerosal Price is obtained through a variety of data sources. The Thimerosal Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Thimerosal manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Thimerosal, including repackagers and relabelers. The FDA regulates Thimerosal manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Thimerosal API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Thimerosal supplier is an individual or a company that provides Thimerosal active pharmaceutical ingredient (API) or Thimerosal finished formulations upon request. The Thimerosal suppliers may include Thimerosal API manufacturers, exporters, distributors and traders.
Thimerosal Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Thimerosal GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Thimerosal GMP manufacturer or Thimerosal GMP API supplier for your needs.
A Thimerosal CoA (Certificate of Analysis) is a formal document that attests to Thimerosal's compliance with Thimerosal specifications and serves as a tool for batch-level quality control.
Thimerosal CoA mostly includes findings from lab analyses of a specific batch. For each Thimerosal CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Thimerosal may be tested according to a variety of international standards, such as European Pharmacopoeia (Thimerosal EP), Thimerosal JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Thimerosal USP).
