



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
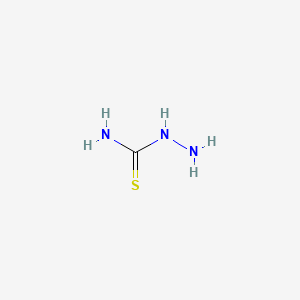

1. N-aminothiourea
2. Thiocarbamylhydrazine
3. Thiosemicarbazide Hydrochloride
4. Thiosemicarbazide Monohydrochloride
1. 79-19-6
2. Hydrazinecarbothioamide
3. N-aminothiourea
4. Aminothiourea
5. 1-aminothiourea
6. Thiocarbamylhydrazine
7. Isothiosemicarbazide
8. 2-thiosemicarbazide
9. 3-thiosemicarbazide
10. Thiocarbamoylhydrazine
11. Semicarbazide, Thio-
12. Aminothio-urea
13. Thiocarbamoyl Hydrazide
14. 1-amino-2-thiourea
15. Semicarbazide, 3-thio-
16. Usaf Ek-1275
17. Rcra Waste Number P116
18. Nsc 2213
19. Mfcd00007620
20. Thiosemicarbazine
21. Tsz
22. Chembl256250
23. 6056o8w6et
24. Nsc-2213
25. Wln: Zmyzus
26. Nsc 2213; Nsc 31792
27. Dsstox_cid_1346
28. Dsstox_rid_76099
29. Dsstox_gsid_21346
30. Isothiosemicarbazide (van)
31. Cas-79-19-6
32. Ccris 1416
33. Hsdb 6050
34. Einecs 201-184-7
35. Rcra Waste No. P116
36. Aminohydrazinomethane-1-thione
37. Aminoisothiourea
38. Unii-6056o8w6et
39. Ai3-16319
40. 1-azanylthiourea
41. Thio-semicarbazide
42. H2nnhcsnh2
43. Thiosemicarbazide, 98%
44. Thiosemicarbazide, 99%
45. (aminothioxomethyl)hydrazine
46. (aminothioxomethyl)-hydrazine
47. Thiosemicarbazide [mi]
48. Dtxsid9021346
49. Thiosemicarbazide, P.a., 98%
50. Chebi:49929
51. Nsc2213
52. 4-(t-butyl)-2-ethoxybenzoicacid
53. Hy-y0032
54. Nsc31792
55. Str00427
56. Zinc8830546
57. 1-amino-2-thiourea [hsdb]
58. Tox21_201348
59. Tox21_302983
60. Bdbm50236982
61. Nsc-31792
62. Stl194285
63. Akos000269047
64. Thiosemicarbazide, Puriss. P.a., 98%
65. Ncgc00091884-01
66. Ncgc00091884-02
67. Ncgc00256506-01
68. Ncgc00258900-01
69. Sy001634
70. Db-030119
71. Thiosemicarbazide, Purum, >=98.0% (rt)
72. Am20100785
73. Cs-0008325
74. Ft-0657078
75. T0221
76. A839613
77. W-104264
78. Q16295007
79. F1908-0101
| Molecular Weight | 91.14 g/mol |
|---|---|
| Molecular Formula | CH5N3S |
| XLogP3 | -1.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 91.02041835 g/mol |
| Monoisotopic Mass | 91.02041835 g/mol |
| Topological Polar Surface Area | 96.2 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 42.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Convulsants
Substances that act in the brain stem or spinal cord to produce tonic or clonic convulsions, often by removing normal inhibitory tone. They were formerly used to stimulate respiration or as antidotes to barbiturate overdose. They are now most commonly used as experimental tools. (See all compounds classified as Convulsants.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Glutamic acid decarboxylase activity in mouse brain homogenates was reduced after pretreatment with thiosemicarbazide.
PMID:497036 Sawaya C et al; Biochem pharmacol 28 (18): 2854-6 (1979)


