



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
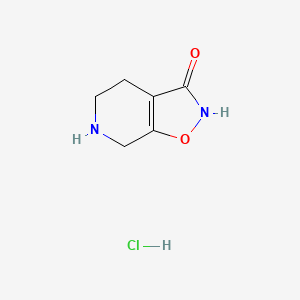

1. Thip Hydrochloride
2. 85118-33-8
3. Gaboxadol (hydrochloride)
4. Gaboxadol Hcl
5. 478rvh3tvd
6. 85118-33-8 (hcl)
7. 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol Hydrochloride
8. 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3(2h)-one Monohydrochloride
9. 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3(2h)-one Hydrochloride
10. 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3(2h)-one Monohydrochloride
11. 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol Hcl, Gaboxadol-hcl
12. Lu 02-030 (hydrochloride); Thip (hydrochloride)
13. Sr-01000075651
14. Unii-478rvh3tvd
15. Einecs 285-687-7
16. Thip Hcl
17. Thip (hydrochloride)
18. (thip)
19. Mls002154080
20. Spectrum1503648
21. Lu 02-030 (hydrochloride)
22. Chembl1255746
23. Dtxsid90234251
24. Hms1571c05
25. Pharmakon1600-01503648
26. Bcp16610
27. Gaboxadol Hydrochloride [mi]
28. Tox21_501233
29. Ccg-39368
30. Mfcd00055180
31. Nsc759585
32. Akos024015212
33. Lp01233
34. Ncgc00094475-01
35. Ncgc00094475-02
36. Ncgc00094475-03
37. Ncgc00094475-04
38. Ncgc00261918-01
39. As-53745
40. Bp-12453
41. Hy-10233
42. Smr000875361
43. J Med Chem 26: 895 (1983)
44. B6460
45. Cs-0002508
46. Eu-0101233
47. G0405
48. T-101
49. P17040
50. Gaboxadol Hydrochloride, Solid, >=98% (hplc)
51. Sr-01000075651-1
52. Sr-01000075651-3
53. Sr-01000075651-6
54. Sr-01000075651-7
55. Q27259031
56. 9-oxa-3,8-diazabicyclo[4.3.0]non-10-en-7-one Hcl
57. 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3(2h)-onehydrochloride
58. 4,5,6,7-tetrahydro-[1,2]oxazolo[5,4-c]pyridin-3-one;hydrochloride
59. Isoxazolo(5,4-c)pyridin-3(2h)-one, 4,5,6,7-tetrahydro-, Hydrochloride (1:1)
60. Isoxazolo(5,4-c)pyridin-3(2h)-one, 4,5,6,7-tetrahydro-, Monohydrochloride
| Molecular Weight | 176.60 g/mol |
|---|---|
| Molecular Formula | C6H9ClN2O2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 176.0352552 g/mol |
| Monoisotopic Mass | 176.0352552 g/mol |
| Topological Polar Surface Area | 50.4 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 210 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |


