
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
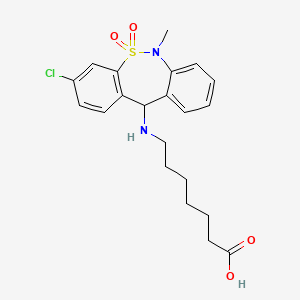

1. (3-chloro-6-methyl-5,5-dioxo-6,11-dihydrodibenzo(c,f)(1,2)thiazepin-11-yl)-7-aminoheptanoic Acid
2. Coaxil
3. Stablon
4. Tianeptine, (+-)-isomer
5. Tianeptine, Monosodium Salt
6. Tianeptine, Monosodium Salt, (+-)-isomer
1. 66981-73-5
2. 72797-41-2
3. Tianeptine [inn]
4. 7-[(3-chloro-6-methyl-5,5-dioxo-11h-benzo[c][2,1]benzothiazepin-11-yl)amino]heptanoic Acid
5. (+)-tianeptine
6. (-)-tianeptine
7. Tianeptine (inn)
8. Tianeptine, (+)-
9. Tianeptine, (-)-
10. Aku7qfl9zt
11. Tpi-1062
12. Stablon (tn)
13. Jnj-39823277
14. 0t493yfu8o
15. Xv6773012i
16. 7-[(3-chloro-6-methyl-5,5-dioxido-6,11-dihydrodibenzo[c,f][1,2]thiazepin-11-yl)amino]heptanoic Acid
17. Tianeptina
18. S-1574
19. Tianeptine Acid
20. S-16190
21. S-16191
22. 191172-75-5
23. 1159812-13-1
24. 169293-31-6
25. 7-((3-chloro-6-methyl-5,5-dioxido-6,11-dihydrodibenzo[c,f][1,2]thiazepin-11-yl)amino)heptanoic Acid
26. 7-[(3-chloro-6,11-dihydro-6-methyl-5,5-dioxidodibenzo[c,f][1,2]thiazepin-11-yl)amino]heptanoic Acid
27. Heptanoic Acid, 7-((3-chloro-6,11-dihydro-6-methyl-5,5-dioxidodibenzo(c,f)(1,2)thiazepin-11-yl)amino)-, (+)-
28. Heptanoic Acid, 7-((3-chloro-6,11-dihydro-6-methyl-5,5-dioxidodibenzo(c,f)(1,2)thiazepin-11-yl)amino)-, (-)-
29. Heptanoic Acid, 7-((3-chloro-6,11-dihydro-6-methyldibenzo(c,f)(1,2)thiazepin-11-yl)amino)-, S,s-dioxide, (+)-
30. Tianeptina [inn-spanish]
31. Unii-0t493yfu8o
32. Tianeptineacid
33. (s)-tianeptine
34. Einecs 276-851-9
35. Tianeptine [mi]
36. Unii-aku7qfl9zt
37. Tianeptine [who-dd]
38. Schembl49293
39. (1)-7-((3-chloro-6,11-dihydro-6-methyldibenzo(c,f)(1,2)thiazepin-11-yl)amino)heptanoic Acid S,s-dioxide
40. Mls006010111
41. Gtpl7558
42. Tianeptine [nflis-drug]
43. Chembl1289110
44. Dtxsid7048295
45. Unii-xv6773012i
46. Chebi:91749
47. Chebi:190006
48. Chebi:190008
49. Hms3886a13
50. Bcp10101
51. Ex-a2724
52. Mfcd00865376
53. S5087
54. Zb1716
55. Akos015900590
56. Ac-2091
57. Ccg-269088
58. Cs-0433
59. Db09289
60. Ks-5099
61. (3-chloro-6-methyl-5,5-dioxo-6,11-dihydrodibenzo(c,f)(1,2)thiazepin-11-yl)-7-aminoheptanoic Acid
62. Hy-90003
63. Smr004701247
64. Ft-0630770
65. Ft-0675218
66. 23t172
67. C76315
68. D02575
69. A835601
70. A914696
71. Q424260
72. Q-100759
73. Brd-a53077924-236-01-4
74. 7-((3-chloro-6,11-dihydro-6-methyldibenzo(c,f)(1,2)thiazepin-11-yl)amino)heptanoic Acid S,s-dioxide
75. 7-[(3-chloro-6-methyl-5,5-dioxo-6,11-dihydro-5h-5lambda(6)-dibenzo[c,f][1,2]thiazepin-11-yl)amino]heptanoic Acid
76. 7-[(6-chloro-10-methyl-9,9-dioxo-9$l^{6}-thia-10-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(15),3(8),4,6,11,13-hexaen-2-yl)amino]heptanoic Acid
77. 7-[[3-chloranyl-6-methyl-5,5-bis(oxidanylidene)-11h-benzo[c][2,1]benzothiazepin-11-yl]amino]heptanoic Acid
78. 7-{[(11s)-3-chloro-6-methyl-5,5-dioxido-6,11-dihydrodibenzo[c,f][1,2]thiazepin-11-yl]amino}heptanoic Acid
| Molecular Weight | 437.0 g/mol |
|---|---|
| Molecular Formula | C21H25ClN2O4S |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Exact Mass | 436.1223562 g/mol |
| Monoisotopic Mass | 436.1223562 g/mol |
| Topological Polar Surface Area | 95.1 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 654 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used primarily in the treatment of major depressive disorder and anxiety. It is currently being studied for fibromyalgia pain treatment.
Analyses in large-scale epidemiologic surveys have shown that the anxiety disorders are widely comorbid with major depression. This makes antidepressant with anxiolytic properties particularly unique and attractive. Tianeptine is effective in reducing depressive symptoms in mild to severe major depressive disorder and also alleviates anxious symptoms associated with depression without the need for coadministration of an anti-anxiety medication. These findings, however, are met with controversial data. In a study of healthy volunteers, Tianeptine-treated subjects were less accurate at identifying facial expressions, suggesting a lack of improvement in the psychomotor symptoms of depression. The tianeptine group also showed reduced memory and reduced attentional vigilance to various stimuli.
Antidepressive Agents, Tricyclic
Substances that contain a fused three-ring moiety and are used in the treatment of depression. These drugs block the uptake of norepinephrine and serotonin into axon terminals and may block some subtypes of serotonin, adrenergic, and histamine receptors. However, the mechanism of their antidepressant effects is not clear because the therapeutic effects usually take weeks to develop and may reflect compensatory changes in the central nervous system. (See all compounds classified as Antidepressive Agents, Tricyclic.)
N06AX14
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AX - Other antidepressants
N06AX14 - Tianeptine
Absorption
Well absorbed, approximately 99% bioavailability.
Route of Elimination
Eliminated with bile as glucuronide and glutamine conjugates.
Volume of Distribution
0.8 L/kg (0.77 +/- 0.31 L/kg)
Clearance
Rapidly cleared by the kidneys.
Tianeptine is metabolized primarily by beta-oxidation of its heptanoic side chain. The metabolism of tianeptine was studied after a one-time oral administration of radioisotopically (14C) labeled compound to healthy male volunteers. After 1 week, approximately 66% of the dose was eliminated by the kidneys (55% elimination during the first 24 hr). After 24h, unchanged drug 3% of the drug was found unchanged in the urine. Three major metabolites result from beta-oxidation of Tianeptine. The metabolite profiles of tianeptine in feces and plasma were found to be qualitatively similar to that in urine.
Approximately 2.5 h
Recent studies suggest that tianeptine acts as a full agonist at the mu-type opioid receptor (MOR),. The mu opioid receptors are currently being studied as effective targets for antidepressant therapies. It is believed that the clinical effects of tianeptine are owed to its modulation of these receptors. In addition to its actions on the opioid receptor, previous studies have owed its action to its effect on the serotonin receptor,, dopamine (D2/3) receptors, and glutamate receptors,, as discussed below: Tianeptine has challenged the monoaminergic hypothesis of depression, as well as the widely supported monoaminergic mechanisms whereby the action of most known antidepressants have been explained. Specifically, this drug is thought to persistently alter glutamate receptor bursting of the hippocampal CA3 commissural associational synapse. Current research suggests that tianeptine produces its antidepressant effects through the modulation of glutamate receptor activity (for example, AMPA receptors and NMDA receptors) and affect the release of brain-derived neurotrophic factor (BDNF), which impacts neural plasticity. More recent studies by support the role of tianeptine in the modulation of glutaminergic activity in the amygdala, the emotional region of the brain associated with memories. Tianeptine reduces the hypothalamic-pituitary-adrenal response to stress, and thus prevents stress-related behavioral issues. In rodents, the stress of acute restraint increases extracellular levels of glutamate in the basolateral amygdala an effect that was inhibited by tianeptine. Interestingly, the SSRI fluoxetine increased extracellular glutamate levels in the basolateral amygdala regardless of stress conditions. These data demonstrate that the mechanism of action of tianeptine is distinct from SSRIs and support the hypothesis that the mechanism of action of tianeptine relates to alteration of glutaminergic activity in the amygdala and the hippocampus. In addition to the above mechanisms, tianeptine is a unique antidepressant and anxiolytic medication that stimulates the uptake of serotonin (5-hydroxytryptamine; 5-HT), and 5-hydroxyindoleacetic acid (5-HIAA) in brain tissue. Although the monoaminergic neurotransmitters serotonin (5-HT), noradrenaline (NA) and dopamine (DA) are proven to be related to the occurrence of depressive disorders, it is now recognized that monoamine deficits are not sufficient to explain the mechanism of action of antidepressant medications.
Market Place

ANALYTICAL

ABOUT THIS PAGE
25
PharmaCompass offers a list of Tianeptine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Tianeptine manufacturer or Tianeptine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Tianeptine manufacturer or Tianeptine supplier.
PharmaCompass also assists you with knowing the Tianeptine API Price utilized in the formulation of products. Tianeptine API Price is not always fixed or binding as the Tianeptine Price is obtained through a variety of data sources. The Tianeptine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tianeptine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tianeptine, including repackagers and relabelers. The FDA regulates Tianeptine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tianeptine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Tianeptine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Tianeptine supplier is an individual or a company that provides Tianeptine active pharmaceutical ingredient (API) or Tianeptine finished formulations upon request. The Tianeptine suppliers may include Tianeptine API manufacturers, exporters, distributors and traders.
click here to find a list of Tianeptine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Tianeptine DMF (Drug Master File) is a document detailing the whole manufacturing process of Tianeptine active pharmaceutical ingredient (API) in detail. Different forms of Tianeptine DMFs exist exist since differing nations have different regulations, such as Tianeptine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Tianeptine DMF submitted to regulatory agencies in the US is known as a USDMF. Tianeptine USDMF includes data on Tianeptine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Tianeptine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Tianeptine suppliers with USDMF on PharmaCompass.
Tianeptine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tianeptine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tianeptine GMP manufacturer or Tianeptine GMP API supplier for your needs.
A Tianeptine CoA (Certificate of Analysis) is a formal document that attests to Tianeptine's compliance with Tianeptine specifications and serves as a tool for batch-level quality control.
Tianeptine CoA mostly includes findings from lab analyses of a specific batch. For each Tianeptine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tianeptine may be tested according to a variety of international standards, such as European Pharmacopoeia (Tianeptine EP), Tianeptine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tianeptine USP).
