
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
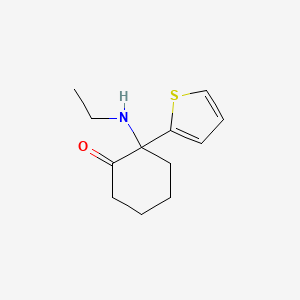

1. 2-(ethylamino)-2-(2-thienyl)cyclohexanone
2. Ci 634
3. Ci-634
4. Ci634
5. Hydrochloride, Tiletamine
6. Tiletamine Hydrochloride
1. 14176-49-9
2. 2-(ethylamino)-2-(2-thienyl)cyclohexanone
3. Cyclohexanone, 2-(ethylamino)-2-(2-thienyl)-
4. 2-(ethylamino)-2-thiophen-2-ylcyclohexan-1-one
5. 14176-49-9 (free Base)
6. Tiletamine (inn)
7. 2yfc543249
8. Tiletamine [inn]
9. Tiletamina
10. Tiletaminum
11. Tiletamine [inn:ban]
12. Tiletaminum [inn-latin]
13. Tiletamina [inn-spanish]
14. Einecs 238-031-9
15. Unii-2yfc543249
16. Hsdb 7638
17. Tiletamine [mi]
18. Prestwick0_001022
19. Prestwick1_001022
20. Prestwick2_001022
21. Prestwick3_001022
22. Tiletamine [hsdb]
23. Tiletamine [who-dd]
24. Bspbio_001203
25. Schembl115212
26. Spbio_003064
27. Bpbio1_001325
28. Chembl2110703
29. Dtxsid5048552
30. Chebi:94356
31. Akos005066229
32. Db11549
33. Ncgc00179264-01
34. Ncgc00179264-03
35. Ac-23715
36. Da-10477
37. Ab00514642
38. Ft-0756967
39. 2-(ethylamino)-2-(2-thienyl)cyclohexanone #
40. D08596
41. 2-(ethylamino)-2-thiophen-2-yl-1-cyclohexanone
42. Ab00514642_02
43. 2-(ethylamino)-2-thiophen-2-yl-cyclohexan-1-one
44. A807808
45. J-524970
46. Q2083144
47. Brd-a30582499-003-01-0
| Molecular Weight | 223.34 g/mol |
|---|---|
| Molecular Formula | C12H17NOS |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 223.10308534 g/mol |
| Monoisotopic Mass | 223.10308534 g/mol |
| Topological Polar Surface Area | 57.3 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 244 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/VET/ This study is the first to compare the anesthetic effects of two cyclohexamines on free-ranging subantarctic fur seal (Arctocephalus tropicalis) females. From April to July 1999, 107 females were immobilized for tooth extraction and blood sampling, using either ketamine (Ketalar, n = 58) alone or tiletamine-zolazepam (Zoletil 100, n = 49) mixture. Animals were injected intramuscularly at mean doses of 2.1 mg/kg for ketamine and 1.1 mg/kg for tiletamine-zolazepam mixture. Individual response to both drugs was highly variable. The dosage required to achieve a satisfactory level of anesthesia was smaller for subantarctic fur seals than for most other species of seals and was less for animals in better body condition. Few side effects were observed during the trials, aside from mild tremors caused by ketamine, and respiratory depression or prolonged apnea caused by tiletamine-zolazepam. ...
PMID:12528456 Dabin W et al; J Wildl Dis 38 (4): 846-50 (2002)
Telazol; /mixture of/ Tiletamine HCl & Zolazapam HCl /is a/ veterinary pharmaceutical /used as a/ general anesthetic. /Telazol/
Fort Dodge; Material Safety Data Sheet (MSDS) on Telazol p.1 (2002) Available from https://www.accessbutler.com/msdsimages/A0000865.pdf as of November 19, 2008
/VET/ Intramuscular injection of tiletamine-zolazepam and xylazine is commonly used as a preanesthetic for veterinary surgical procedures and for short-term restraint. However, this combination can have marked cardiodepressive and hypothermic effects that persist for hours to days. Here ... a case report of these effects in a swine heart failure model /is presented/.
PMID:17994676 Lefkov SH, Mussig D; J Am Assoc Lab Anim Sci 46 (6): 63-4 (2007)
/VET/ Medetomidine (0.02-0.06 mg/kg) in combination with zolazepam-tiletamine (0.8-2.3 mg/kg) were evaluated for reversible anesthesia in four species of Southeast Asian primates: Bornean orangutan (Pongo pygmaeus pygmaeus), Bornean gibbon (Hylobates muelleri), long-tailed macaque (Macaca fascicularis), and pig-tailed macaque (Macaca nemestrina). Twenty-three anesthetic procedures of captive-held and free-ranging primates were studied in Sabah, Malaysia. The induction was smooth and rapid. Respiratory and heart rates were stable throughout anesthesia, whereas body temperature and systolic arterial blood pressure decreased significantly. Atipamezole at five times the medetomidine dose effectively reversed anesthesia, with first signs of recovery within 3-27 min.
PMID:17315446 Fahlman A et al; J Zoo Wildl Med 37 (4): 558-61
For more Therapeutic Uses (Complete) data for TILETAMINE (21 total), please visit the HSDB record page.
Two commonly used anesthetic combinations, ketamine acepromazine and tiletamine-zolazepam, were compared to identify their effects upon body temperature in cynomolgus macaques. 30 cynomolgus macaques previously implanted with subcutaneous telemetry devices /were allocated/ into two groups of 15 animals. Baseline temperature data were collected for 3 days before administering anesthesia to establish normal diurnal temperature patterns for each monkey. Each group then was anesthetized with either ketamine-acepromazine or tiletamine-zolazepam, and their body temperatures were recorded at 15-min intervals. Both groups had marked decreases in body temperature, with the greatest decreases in the tiletamine-zolazepam group. In addition, both groups had notable post-anesthesia elevations in body temperature that often lasted for more than 24 hr postinduction.
PMID:11958603 Lopez KR et al; Contemp Top Lab Anim Sci 41 (2): 47-50 (2002)
... Tiletamine and zolazepam were given to five elephant seals and one leopard seal. Two of the elephant seals and the leopard seal died from unknown causes...
PMID:1750175 Mitchell PJ, Burton HR;Vet Rec 129 (15): 332-6 (1991)
/VET/ The efficacy of ketamine-xylazine /was compared/ to tiletamine-zolazepam-xylazine for producing surgical anesthesia in rabbits. Four of six rabbits receiving ketamine-xylazine and all of the 12 animals given tiletamine-zolazepam-xylazine were anesthetized successfully. The mean surgical anesthesia time in the ketamine-xylazine group was 35 +/- 6 minutes as compared to the tiletamine-zolazepam-xylazine group, 72 +/- 8 minutes (p less than 0.05). There was no significant difference in the interval between the injection of the different anesthetic mixtures and the loss of either the righting reflex, the jaw reflex or the toe web pinch reflex. Respiratory rates and arterial oxygen partial pressure were higher in the ketamine-xylazine group (p < 0.05). However, in both groups arterial blood pressure and arterial PO2 were lowered, while arterial PCO2 was elevated. No nephrotoxicity occurred. Tiletamine-zolazepam-xylazine provides effective surgical anesthesia in rabbits and in many cases may be preferable to conventional ketamine-xylazine regimen.
PMID:1849587 Popilskis SJ et al; Lab Anim Sci 41 (1): 51-3 (1991)
Hypoxemia is a commonly observed complication during the chemical immobilization of wild ruminants. If severe and left untreated, it can predispose animals to arrhythmias, organ failure, and capture myopathy. The following prospective study was designed to measure the degree of hypoxemia in wapiti /Cervus canadensis (North American elk)/ that were immobilized with a combination of xylazine and tiletamine-zolazepam and to assess the response to nasal oxygen therapy. ... All wapiti exhibited mild to marked hypoxemia (PaO2 = 43 +/- 11.8 mmHg) prior to treatment and showed marked improvement after 5 minutes of nasal insufflation of oxygen at 10 L/min (PaO2 = 207 +/- 60 mmHg). This inexpensive, noninvasive technique has great benefit in treating clinical hypoxemia under field conditions, and ... nasal insufflation of oxygen /was recommended to/ be implemented during xylazine-tiletamine-zolazepam-induced immobilization of wapiti and other wild ruminants.
PMID:11708204 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1476651 Read MR et al; Can Vet J 42 (11): 861-4 (2001)
Captive cheetah (Acinonyx jubatus) scheduled for either general health examination or dental surgery were immobilized with combinations of medetomidine-ketamine (K/DET, n = 19), midazolam-ketamine (K/MID, n = 4) or medetomidine-tiletamine-zolazepam (Z/DET, n = 5). Induction time and arterial blood pressure was not statistically significantly (P > 0.05) different between treatment groups. Transient seizures were observed in the K/DET treated animals during induction. Hypertension was present in all groups during anesthesia with mean (+/- SD) systolic pressure of 30.7 +/- 5.0 kPa for the K/DET group, 27.7 +/- 2.7 kPa for the K/MID group, and 33.1 +/- 4.6 kPa for the Z/DET group. Heart rate was statistically significantly (P < 0.05) lower in the K/DET group (69 +/- 13.2 beats/min) compared to the K/MID group (97 +/- 22.6 beats/min), and ventilation rate was statistically significantly (P < 0.05) lower in the K/MID group (15 +/- 0.0 breaths/min) compared with the K/DET group (21 +/- 4.6). A metabolic acidosis and hypoxia were observed during anesthesia when breathing air. Oxygen (O2) administration resulted in a statistically significant (P < 0.05) increase in the arterial partial pressure of carbon dioxide (hypercapnea), arterial partial pressure of O2, and % oxyhemoglobin saturation.
PMID:17458346 Stegmann GF, Jago M; J S Afr Vet Assoc. 2006 Dec;77(4):205-9
Anesthetics, Dissociative
Intravenous anesthetics that induce a state of sedation, immobility, amnesia, and marked analgesia. Subjects may experience a strong feeling of dissociation from the environment. The condition produced is similar to NEUROLEPTANALGESIA, but is brought about by the administration of a single drug. (From Gilman et al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed) (See all compounds classified as Anesthetics, Dissociative.)
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
A pharmacokinetic and tissue residue study was conducted to assess the risks associated with human consumption of polar bears in arctic Canada that have been exposed to the immobilizing drug Telazol, a mixture of tiletamine hydrochloride and zolazepam hydrochloride. Twenty-two bears were remotely injected with about 10 mg/kg of Telazol. Following immobilization, serum samples were collected serially at regular intervals until the bears awakened. Sixteen of the bears were relocated and killed under permit by local hunters at various times from 0.5 to 11 days after dosing. Serum, kidney, muscle and adipose tissue samples were collected immediately after death. ... In addition, the serum and tissue samples collected at the time of death were analyzed for both parent drugs, for one metabolite of tiletamine (CI-398), and for three metabolites of zolazepam (metabolites 1, 2 and 4). A one-compartment model with first-order absorption and elimination best fit the time-series data for the drugs in serum during the immobilization period. This model gave half-lives (mean +/- SE) for tiletamine and zolazepam of 1.8+/-0.2 hr and 1.2+/-0.08 hr, respectively, clearance values of 2.1+/-0.3 l x hr(-1) x kg(-1) and 1.1+/-0.1 l x hr(-1) x kg(-1), and volumes of distribution of 5.2+/-0.6 L/kg and 1.8+/-0.2 L/kg. The concentrations of both drugs and their metabolites declined rapidly to trace levels by 24 hr post-dosing, although extremely low concentrations of some metabolites were encountered sporadically over the entire sampling period. In particular, zolazepam metabolite 2, remained detectable in fat and muscle tissue at the end of the study, 11 days after dosing. It was concluded that during immobilization, both tiletamine and zolazepam levels decline rapidly in a monoexponential fashion, and their pharmacokinetic parameters in polar bears are similar to those observed in other species. Tissue levels of the drugs and their metabolites declined sufficiently rapidly that individuals eating meat from exposed bears would be unlikely to experience pharmacological effects from the drugs. Nevertheless, slight exposure to the drugs and/or their metabolites might be possible for an indeterminate time after dosing.
PMID:11085426 Semple HA et al; J Wildl Dis 36 (4): 653-62 (2000)
Neurochemical interactions of tiletamine, a potent phencyclidine (PCP) receptor ligand, with the N-methyl-D-aspartate (NMDA)-coupled and -uncoupled PCP recognition sites were examined. Tiletamine potently displaced the binding of [3H]1-(2-thienyl)cyclohexylpiperidine with an IC50 of 79 nM without affecting sigma-, glycine, glutamate, kainate, quisqualate, or dopamine (DA) receptors. Like other PCP ligands acting via the NMDA-coupled PCP recognition sites, tiletamine decreased basal, harmaline-, and D-serine-mediated increases in cyclic GMP levels and induced stereotypy and ataxia. Tiletamine was nearly five times more potent than PCP at inhibiting the binding of 3-hydroxy[3H]PCP to its high-affinity NMDA-uncoupled PCP recognition sites. However, following parenteral administration, dizocilpine maleate (MK-801), ketamine, PCP, dexoxadrol, and 1-(2-thienyl)cyclohexylpiperidine HCl, but not tiletamine, increased rat pyriform cortical DA metabolism and/or release, a response modulated by the NMDA-uncoupled PCP recognition sites. Pretreatment with tiletamine did not attenuate the MK-801-induced increases in rat pyriform cortical DA metabolism, a result suggesting that tiletamine is not a partial agonist of the NMDA-uncoupled PCP recognition sites in this region. However, following intracerebroventricular administration (100-500 ug/rat), tiletamine increased pyriform cortical DA metabolism with a bell-shaped dose-response curve. These data indicate a differential interaction of tiletamine with the NMDA-coupled and -uncoupled PCP recognition sites. The paradoxical effects of tiletamine suggest that tiletamine might activate receptor(s) or neuronal pathways of unknown pharmacology.
PMID:1847186 Rao TS et al; J Neurochem 56 (3): 890-7 (1991)
...This study shows tiletamine to be similar to ketamine and to phencyclidine, agents known to interact with the N-methyl-D,L-aspartate (NMA) receptor. Effects of tiletamine on synaptic transmission and on direct excitatory responses to exogenous amino acids were examined in rat hippocampal and striatal slices. In striatal slices, tiletamine inhibited the NMA-mediated, but not the spontaneous, release of [3H]acetylcholine, with an IC50 of 70 nM. In hippocampal CA1 cells, 3 uM tiletamine in the perfusate reversibly blocked the intracellularly recorded responses to ionophoretically applied NMA, but not to glutamate, quisqualate and kainate. Tiletamine, 3 to 100 uM, had no effect on the orthodromically elicited excitatory postsynaptic potential, action potential amplitude or duration, resting membrane potential, or input resistance. In Mg++-free perfusate, the excitatory postsynaptic potential was greatly augmented to give a paroxysmal depolarization shift and was reversibly blocked by 10 uM tiletamine. /These/ results show that tiletamine is a potent and reversible antagonist of NMA-mediated responses without itself having major effects in low concentrations on normal membrane and synaptic pyramidal cell properties.
PMID:3320347 French-Mullen JM et al; J Pharmacol Exp Ther 243 (3): 915-20 (1987)
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
55
PharmaCompass offers a list of Tiletamine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Tiletamine manufacturer or Tiletamine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Tiletamine manufacturer or Tiletamine supplier.
PharmaCompass also assists you with knowing the Tiletamine API Price utilized in the formulation of products. Tiletamine API Price is not always fixed or binding as the Tiletamine Price is obtained through a variety of data sources. The Tiletamine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tiletamine Hydrochloride manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tiletamine Hydrochloride, including repackagers and relabelers. The FDA regulates Tiletamine Hydrochloride manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tiletamine Hydrochloride API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Tiletamine Hydrochloride manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Tiletamine Hydrochloride supplier is an individual or a company that provides Tiletamine Hydrochloride active pharmaceutical ingredient (API) or Tiletamine Hydrochloride finished formulations upon request. The Tiletamine Hydrochloride suppliers may include Tiletamine Hydrochloride API manufacturers, exporters, distributors and traders.
click here to find a list of Tiletamine Hydrochloride suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Tiletamine Hydrochloride as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Tiletamine Hydrochloride API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Tiletamine Hydrochloride as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Tiletamine Hydrochloride and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Tiletamine Hydrochloride NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Tiletamine Hydrochloride suppliers with NDC on PharmaCompass.
Tiletamine Hydrochloride Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tiletamine Hydrochloride GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tiletamine Hydrochloride GMP manufacturer or Tiletamine Hydrochloride GMP API supplier for your needs.
A Tiletamine Hydrochloride CoA (Certificate of Analysis) is a formal document that attests to Tiletamine Hydrochloride's compliance with Tiletamine Hydrochloride specifications and serves as a tool for batch-level quality control.
Tiletamine Hydrochloride CoA mostly includes findings from lab analyses of a specific batch. For each Tiletamine Hydrochloride CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tiletamine Hydrochloride may be tested according to a variety of international standards, such as European Pharmacopoeia (Tiletamine Hydrochloride EP), Tiletamine Hydrochloride JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tiletamine Hydrochloride USP).
