
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
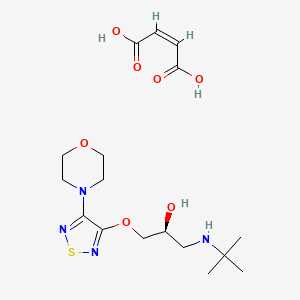

1. (s)-1-((1,1-dimethylethyl)amino)-3-((4-(4-morpholinyl)-1,2,5-thiadazol-3-yl)oxy)-2-propanol
2. 2-propanol, 1-((1,1-dimethylethyl)amino)-3-((4-(4-morpholinyl)-1,2,5-thiadiazol-3-yl)oxy)-, (s)-
3. Blocadren
4. L 714,465
5. L-714,465
6. L714,465
7. Mk 950
8. Mk-950
9. Mk950
10. Optimol
11. Timacar
12. Timolol
13. Timolol Hemihydrate
14. Timolol Maleate, (1:1) Salt
15. Timoptic
16. Timoptol
1. 26921-17-5
2. (s)-timolol Maleate
3. Timolol Maleate Salt
4. Blocadren
5. Timoptic
6. Timolol Hydrogen Maleate
7. Istalol
8. (s)-timolol (maleate)
9. (s)-timolol Hydrogen Maleate
10. Timoptic-xe
11. Ophtamolol
12. Aquanil
13. Mk 950
14. Timolol (as Maleate)
15. Timolol Hydrogen Maleate Salt
16. Timolol Maleate, S-enantiomer
17. Wp-934
18. Mls000028539
19. Chebi:9600
20. 60469-65-0
21. P8y54f701r
22. (-)-1-(tert-butylamino)-3-((4-morpholino-1,2,5-thiadiazol-3-yl)oxy)-2-propanol Maleate
23. Nsc-757351
24. (-)-1-(tert-butylamino)-3-((4-morpholino-1,2,5-thiadiazol-3-yl)oxy)-2-propanol Maleate (1:1) (salt)
25. (z)-but-2-enedioic Acid;(2s)-1-(tert-butylamino)-3-[(4-morpholin-4-yl-1,2,5-thiadiazol-3-yl)oxy]propan-2-ol
26. 2-propanol, 1-((1,1-dimethylethyl)amino)-3-((4-(4-morpholinyl)-1,2,5-thiadiazol-3-yl)oxy)-, (s)-, (z)-2-butenedioate (1:1) (salt)
27. Smr000058305
28. Timacor
29. Betime
30. L-timolol Maleate
31. Timoptic In Ocudose
32. Mfcd00058356
33. 2-propanol, 1-[(1,1-dimethylethyl)amino]-3-[[4-(4-morpholinyl)-1,2,5-thiadiazol-3-yl]oxy]-, (2s)-, (2z)-2-butenedioate (salt)
34. Ccris 1057
35. Einecs 248-111-5
36. Cas-26921-17-5
37. (s)-(-)-timolol Maleate
38. Timololmaleate
39. Timololi Maleas
40. Unii-p8y54f701r
41. S-timolol Maleate
42. Blocadren (tn)
43. Timolol Maleate [usan:usp:jan]
44. Timoptic (tn)
45. Einecs 262-251-4
46. Istalol (tn)
47. Bimatoprost / Timolol
48. Timolol Hydrogenmaleate
49. Opera_id_1256
50. Dsstox_cid_27504
51. Dsstox_rid_82384
52. Dsstox_gsid_47504
53. Schembl26233
54. Mls001076135
55. Timolol Maleate [jan]
56. Timolol Maleate (jp17/usp)
57. Timolol Maleate [usan]
58. L-714,465 (maleate)
59. Timolol Maleate [vandf]
60. Chembl1200870
61. Dtxsid3047504
62. Timolol Maleate [mart.]
63. Timolol Maleate [usp-rs]
64. Timolol Maleate [who-dd]
65. Timolol Maleate [who-ip]
66. Hms1570n18
67. Hms2097n18
68. Hms2230n19
69. Hms2234f03
70. Hms3411m12
71. Hms3675m12
72. Hms3714n18
73. Hms3885l05
74. Tox21_302468
75. S4123
76. Timolol Maleate [orange Book]
77. Akos024458595
78. Timolol Maleate [ep Monograph]
79. Timolol Maleate [usp Impurity]
80. Ccg-220948
81. Cosopt Component Timolol Maleate
82. Cs-1028
83. Nsc 757351
84. Timolol Maleate [usp Monograph]
85. Timololi Maleas [who-ip Latin]
86. (s)-3-(3-(tert-butylamino)-2-hydroxypropoxy)-4-morpholino-1,2,5-thiadiazole Maleate
87. Duotrav Component Timolol Maleate
88. Ganfort Component Timolol Maleate
89. Xalacom Component Timolol Maleate
90. Combigan Component Timolol Maleate
91. Ncgc00256737-01
92. 2-propanol, 1-(tert-butylamino)-3-((4-morpholino-1,2,5-thiadiazol-3-yl)oxy)-, (-)-, Maleate (1:1) (salt)
93. 2-propanol, 1-[(1,1-dimethylethyl)amino]-3-[[4-(4-morpholinyl)-1,2,5-thiadiazol-3-yl]oxy]-, (2s)-, (2z)-2-butenedioate (1:1) (salt)
94. As-18090
95. Bs164420
96. Hy-17380
97. Smr000653470
98. Timolol Hydrogen Maleate Salt [mi]
99. Timolol Maleate Component Of Cosopt
100. Timolol Maleate Component Of Duotrav
101. Timolol Maleate Component Of Ganfort
102. T2905
103. Timolol Maleate Component Of Combigan
104. Timolol Maleate Salt, >=98% (tlc), Powder
105. A13429
106. D00603
107. F20622
108. Timolol Maleate, S-enantiomer [who-dd]
109. 921t175
110. Sr-01000003102
111. Q-201836
112. Sr-01000003102-3
113. Q27108445
114. Timolol Maleate 1.0 Mg/ml In Methanol (as Free Base)
115. Timolol Maleate, British Pharmacopoeia (bp) Reference Standard
116. Timolol Maleate, European Pharmacopoeia (ep) Reference Standard
117. Timolol Maleate, United States Pharmacopeia (usp) Reference Standard
118. (s)-1-tert-butylamino-3-(4-morpholin-4-yl-[1,2,5]thiadiazol-3-yloxy)-propan-2-ol Maleate
119. 1-( Tert-butylamino)-3-[(4-morpholino-1,2,5-thiadiazol-3-yl)oxy]-2-propanol Maleate
120. Timolol For System Suitability, European Pharmacopoeia (ep) Reference Standard
121. (2s)-1-(tert-butylamino)-3-{[4-(morpholin-4-yl)-1,2,5-thiadiazol-3-yl]oxy}propan-2-ol (2z)-but-2-enedioate
122. (s)-1-[(1,1-dimethylethyl)amino]-3-[[4-(4-morpholinyl)-1,2,5-thiadiazol-3-yl]oxy]-2-propanol Maleate
| Molecular Weight | 432.5 g/mol |
|---|---|
| Molecular Formula | C17H28N4O7S |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 9 |
| Exact Mass | 432.16787042 g/mol |
| Monoisotopic Mass | 432.16787042 g/mol |
| Topological Polar Surface Area | 183 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 429 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 8 | |
|---|---|
| Drug Name | Timoptic-xe |
| Drug Label | TIMOPTIC-XE (timolol maleate ophthalmic solution) is a non-selective beta-adrenergic receptor blocking agent. Its chemical name is (-)-1-(tert-butylamino)-3- [(4-morpholino-1, 2, 5-thiadiazol-3-yl)oxy]-2-propanol maleate (1:1) (salt). Timolol malea... |
| Active Ingredient | Timolol maleate |
| Dosage Form | Solution, gel forming/drops |
| Route | Ophthalmic |
| Strength | eq 0.25% base; eq 0.5% base |
| Market Status | Prescription |
| Company | Valeant Pharms |
| 2 of 8 | |
|---|---|
| Drug Name | Timolol maleate |
| Active Ingredient | Timolol maleate |
| Dosage Form | Solution/drops; Tablet; Solution, gel forming/drops |
| Route | Ophthalmic; Oral |
| Strength | eq 0.25% base; 5mg; 10mg; eq 0.5% base; 20mg |
| Market Status | Prescription |
| Company | Wockhardt; Falcon Pharms; Pacific Pharma; Hi Tech Pharma; Bausch And Lomb; Fdc; Mylan; Akorn |
| 3 of 8 | |
|---|---|
| Drug Name | Timoptic |
| Drug Label | Timolol Maleate Ophthalmic Gel Forming Solution is a non-selective beta-adrenergic receptor blocking agent. Its chemical name is (-)-1-(tert-butylamino)-3- [(4-morpholino-1, 2, 5-thiadiazol-3-yl)oxy]-2-propanol maleate (1:1) (salt). Timolol maleate p... |
| Active Ingredient | Timolol maleate |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | eq 0.25% base; eq 0.5% base |
| Market Status | Prescription |
| Company | Aton |
| 4 of 8 | |
|---|---|
| Drug Name | Timoptic in ocudose |
| PubMed Health | Timolol (Into the eye) |
| Drug Classes | Antiglaucoma |
| Drug Label | TIMOPTIC-XE (timolol maleate ophthalmic solution) is a non-selective beta-adrenergic receptor blocking agent. Its chemical name is (-)-1-(tert-butylamino)-3- [(4-morpholino-1, 2, 5-thiadiazol-3-yl)oxy]-2-propanol maleate (1:1) (salt). Timolol malea... |
| Active Ingredient | Timolol maleate |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | eq 0.25% base; eq 0.5% base |
| Market Status | Prescription |
| Company | Aton |
| 5 of 8 | |
|---|---|
| Drug Name | Timoptic-xe |
| Drug Label | TIMOPTIC-XE (timolol maleate ophthalmic solution) is a non-selective beta-adrenergic receptor blocking agent. Its chemical name is (-)-1-(tert-butylamino)-3- [(4-morpholino-1, 2, 5-thiadiazol-3-yl)oxy]-2-propanol maleate (1:1) (salt). Timolol malea... |
| Active Ingredient | Timolol maleate |
| Dosage Form | Solution, gel forming/drops |
| Route | Ophthalmic |
| Strength | eq 0.25% base; eq 0.5% base |
| Market Status | Prescription |
| Company | Valeant Pharms |
| 6 of 8 | |
|---|---|
| Drug Name | Timolol maleate |
| Active Ingredient | Timolol maleate |
| Dosage Form | Solution/drops; Tablet; Solution, gel forming/drops |
| Route | Ophthalmic; Oral |
| Strength | eq 0.25% base; 5mg; 10mg; eq 0.5% base; 20mg |
| Market Status | Prescription |
| Company | Wockhardt; Falcon Pharms; Pacific Pharma; Hi Tech Pharma; Bausch And Lomb; Fdc; Mylan; Akorn |
| 7 of 8 | |
|---|---|
| Drug Name | Timoptic |
| Drug Label | Timolol Maleate Ophthalmic Gel Forming Solution is a non-selective beta-adrenergic receptor blocking agent. Its chemical name is (-)-1-(tert-butylamino)-3- [(4-morpholino-1, 2, 5-thiadiazol-3-yl)oxy]-2-propanol maleate (1:1) (salt). Timolol maleate p... |
| Active Ingredient | Timolol maleate |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | eq 0.25% base; eq 0.5% base |
| Market Status | Prescription |
| Company | Aton |
| 8 of 8 | |
|---|---|
| Drug Name | Timoptic in ocudose |
| PubMed Health | Timolol (Into the eye) |
| Drug Classes | Antiglaucoma |
| Drug Label | TIMOPTIC-XE (timolol maleate ophthalmic solution) is a non-selective beta-adrenergic receptor blocking agent. Its chemical name is (-)-1-(tert-butylamino)-3- [(4-morpholino-1, 2, 5-thiadiazol-3-yl)oxy]-2-propanol maleate (1:1) (salt). Timolol malea... |
| Active Ingredient | Timolol maleate |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | eq 0.25% base; eq 0.5% base |
| Market Status | Prescription |
| Company | Aton |
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Adrenergic beta-Antagonists
Drugs that bind to but do not activate beta-adrenergic receptors thereby blocking the actions of beta-adrenergic agonists. Adrenergic beta-antagonists are used for treatment of hypertension, cardiac arrhythmias, angina pectoris, glaucoma, migraine headaches, and anxiety. (See all compounds classified as Adrenergic beta-Antagonists.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
 Transo-Pharm GmbH works globally to supply Active Pharmaceutical Ingredients adhering to the highest quality & GMP standards.
Transo-Pharm GmbH works globally to supply Active Pharmaceutical Ingredients adhering to the highest quality & GMP standards.
 Bhavna Laboratories is an API & Intermediate manufacturer focusing on the muscle relaxant & ophthalmic segments.
Bhavna Laboratories is an API & Intermediate manufacturer focusing on the muscle relaxant & ophthalmic segments.
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.

Registration Number : 306MF10098
Registrant's Address : 21,chemin de la Sauvegarde 21 Ecully Parc - CS 33167 69134 Ecully Cedex France
Initial Date of Registration : 2024-07-17
Latest Date of Registration : --
 IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
 Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 6983
Submission : 1987-05-22
Status : Active
Type : II

Certificate Number : CEP 2014-300 - Rev 01
Issue Date : 2023-09-27
Type : Chemical
Substance Number : 572
Status : Valid

Registration Number : 219MF10315
Registrant's Address : 21,chemin de la Sauvegarde 21 Ecully Parc - CS 33167 69134 Ecully Cedex France
Initial Date of Registration : 2007-10-24
Latest Date of Registration : --

NDC Package Code : 51014-7125
Start Marketing Date : 2015-05-27
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Eltek Pharmachem Co., Ltd.
Registration Date : 2023-11-23
Registration Number : 20231123-201-I-665-10
Manufacturer Name : PCAS Finland Oy
Manufacturer Address : Messukentankatu 8, 20210 Turku, Finland

| Available Reg Filing : ASMF |
 Fareva is a one-stop-shop for API/HPAPI, OSD & Sterile products, is USFDA, EMA and PMDA-inspected and has multiple NDA & ANDA filings.
Fareva is a one-stop-shop for API/HPAPI, OSD & Sterile products, is USFDA, EMA and PMDA-inspected and has multiple NDA & ANDA filings.

GDUFA
DMF Review : Reviewed
Rev. Date : 2013-10-05
Pay. Date : 2013-09-26
DMF Number : 15516
Submission : 2001-07-04
Status : Active
Type : II

Certificate Number : CEP 2021-304 - Rev 00
Issue Date : 2024-04-24
Type : Chemical
Substance Number : 572
Status : Valid

Registration Number : 219MF10207
Registrant's Address : NUERNBERGER STRASSE 12, 90537 FEUCHT, GERMANY
Initial Date of Registration : 2007-06-13
Latest Date of Registration : --

NDC Package Code : 46014-1059
Start Marketing Date : 2007-08-02
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT FOR HUMAN PRESCRIPTION COMPOUNDING

Registrant Name : Samoh Pharmaceutical Co., Ltd.
Registration Date : 2012-12-27
Registration Number : 20121227-201-I-91-04
Manufacturer Name : Fareva La Vallee
Manufacturer Address : Zone Industrielle de Blavozy, 928 avenue Lavoisier, 43700 Saint-Germain-Laprade

Date of Issue : 2022-07-08
Valid Till : 2024-11-04
Written Confirmation Number : WC-0432
Address of the Firm :
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
GDUFA
DMF Review : Complete
Rev. Date : 2017-02-27
Pay. Date : 2017-01-23
DMF Number : 29342
Submission : 2015-07-07
Status : Active
Type : II
 Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
GDUFA
DMF Review : Complete
Rev. Date : 2013-10-31
Pay. Date : 2012-12-20
DMF Number : 10690
Submission : 1994-01-25
Status : Active
Type : II
 Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 6983
Submission : 1987-05-22
Status : Active
Type : II
 Fareva is a one-stop-shop for API/HPAPI, OSD & Sterile products, is USFDA, EMA and PMDA-inspected and has multiple NDA & ANDA filings.
Fareva is a one-stop-shop for API/HPAPI, OSD & Sterile products, is USFDA, EMA and PMDA-inspected and has multiple NDA & ANDA filings.
GDUFA
DMF Review : Complete
Rev. Date : 2019-05-13
Pay. Date : 2019-03-28
DMF Number : 32064
Submission : 2019-03-27
Status : Active
Type : II
 Fareva is a one-stop-shop for API/HPAPI, OSD & Sterile products, is USFDA, EMA and PMDA-inspected and has multiple NDA & ANDA filings.
Fareva is a one-stop-shop for API/HPAPI, OSD & Sterile products, is USFDA, EMA and PMDA-inspected and has multiple NDA & ANDA filings.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15580
Submission : 2001-08-10
Status : Active
Type : II
 Fareva is a one-stop-shop for API/HPAPI, OSD & Sterile products, is USFDA, EMA and PMDA-inspected and has multiple NDA & ANDA filings.
Fareva is a one-stop-shop for API/HPAPI, OSD & Sterile products, is USFDA, EMA and PMDA-inspected and has multiple NDA & ANDA filings.
GDUFA
DMF Review : Complete
Rev. Date : 2013-10-05
Pay. Date : 2013-09-26
DMF Number : 15516
Submission : 2001-07-04
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2012-10-25
Pay. Date : 2012-11-27
DMF Number : 9839
Submission : 1992-07-27
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2014-06-16
Pay. Date : 2014-06-09
DMF Number : 6644
Submission : 1986-09-10
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8226
Submission : 1989-09-29
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2012-12-26
Pay. Date : 2012-11-13
DMF Number : 7264
Submission : 1987-12-22
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?