
Synopsis
Synopsis
0
KDMF
0
VMF
0
Canada

0
Australia

0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Bioshik
2. Cp 12,574
3. Cp 12574
4. Cp-12,574
5. Cp-12574
6. Cp12,574
7. Cp12574
8. Fasigin
9. Fasigyn
10. Fasigyne
11. Fasygin
12. Simplotan
13. Tricolam
1. 19387-91-8
2. Tindamax
3. Trimonase
4. Fasigyn
5. Tricolam
6. Fasigin
7. 1-(2-(ethylsulfonyl)ethyl)-2-methyl-5-nitro-1h-imidazole
8. Simplotan
9. 1-(2-ethylsulfonylethyl)-2-methyl-5-nitroimidazole
10. 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitro-1h-imidazole
11. Timidazole
12. Haisigyn
13. Cp-12574
14. 1h-imidazole, 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitro-
15. Cp-12,574
16. 1-(2-(ethylsulfonyl)ethyl)-2-methyl-5-nitroimidazole
17. 1-[2-(ethanesulfonyl)ethyl]-2-methyl-5-nitro-1h-imidazole
18. Tinidazol
19. Chebi:63627
20. 1h-imidazole, 1-(2-(ethylsulfonyl)ethyl)-2-methyl-5-nitro-
21. Nsc-758189
22. Mls000069717
23. 148159-84-6
24. Bioshik
25. 033kf7v46h
26. 1-(2-ethylsulfonylethyl)-2-methyl-5-nitro-imidazole
27. Ncgc00016741-01
28. Tinidazolum
29. Smr000058194
30. Sorquetan
31. Pletil
32. Tinidazole 100 Microg/ml In Acetonitrile
33. Cas-19387-91-8
34. Dsstox_cid_3676
35. Dsstox_rid_77141
36. Dsstox_gsid_23676
37. 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazole
38. Glongyn
39. Amtiba
40. Cp 12574
41. Tinidazol [inn-spanish]
42. Tinidazolum [inn-latin]
43. 1h-imidazole, 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitro-, Radical Ion(1-)
44. Ccris 7621
45. Hsdb 7362
46. Sr-01000000210
47. Einecs 243-014-4
48. Brn 0618182
49. Symplotan
50. Ethyl (2-(2-methyl-5-nitro-1-imidazolyl)ethyl)sulfone
51. Unii-033kf7v46h
52. Fasigyntrade Mark
53. Tindamaxtrade Mark
54. Tinidazole,(s)
55. Simplotantrade Mark
56. Haisigyn (tn)
57. Tindamax (tn)
58. Prestwick_136
59. Cp12574
60. Tinidazole [usan:usp:inn:ban:jan]
61. Spectrum_001266
62. Opera_id_779
63. Specplus_000708
64. Tinidazole [mi]
65. Tinidazole [inn]
66. Tinidazole [jan]
67. Prestwick0_000766
68. Prestwick1_000766
69. Prestwick2_000766
70. Prestwick3_000766
71. Spectrum2_000648
72. Spectrum3_001512
73. Spectrum4_000230
74. Spectrum5_001715
75. Tinidazole [hsdb]
76. Tinidazole [usan]
77. Tinidazole [vandf]
78. Tinidazole [mart.]
79. Chembl1220
80. Tinidazole [usp-rs]
81. Tinidazole [who-dd]
82. Oprea1_342844
83. Schembl21141
84. Bspbio_000812
85. Bspbio_003183
86. Kbiogr_000899
87. Kbioss_001746
88. Methyl-5-nitro-1h-imidazole
89. 5-23-05-00067 (beilstein Handbook Reference)
90. Mls001146883
91. Mls001424192
92. Divk1c_006804
93. Spectrum1502127
94. Spbio_000655
95. Spbio_002751
96. Bpbio1_000894
97. Tinidazole (jp17/usp/inn)
98. 1-(ethylsulfonyl)-2-(2-methyl-5-nitroimidazolyl)ethane
99. Dtxsid4023676
100. Tinidazole [orange Book]
101. Kbio1_001748
102. Kbio2_001746
103. Kbio2_004314
104. Kbio2_006882
105. Kbio3_002683
106. Imidazole, 1-(2-(ethylsulfonyl)ethyl)-2-methyl-5-nitro-
107. Tinidazole [ep Monograph]
108. 1-(2-(ethylsulfonyl)ethyl)-2-
109. Hms1570i14
110. Hms1921l06
111. Hms2052c09
112. Hms2090b14
113. Hms2092d14
114. Hms2097i14
115. Hms2231e23
116. Hms3374p07
117. Hms3394c09
118. Hms3652a08
119. Hms3714i14
120. Pharmakon1600-01502127
121. Tinidazole [usp Monograph]
122. Zinc113446
123. Bcp20694
124. Hy-b0177
125. Tox21 110586
126. Tox21_110586
127. Bbl010767
128. Bdbm50248360
129. Ccg-39907
130. Mfcd00057217
131. Nsc758189
132. S4068
133. Stk801761
134. Akos000747070
135. Tinidazole 100 Microg/ml In Methanol
136. Tox21_110586_1
137. Ac-1618
138. Cs-2055
139. Db00911
140. Ks-5180
141. Mcule 2580495783
142. Nc00346
143. Nsc 758189
144. Ncgc00016741-02
145. Ncgc00016741-03
146. Ncgc00016741-04
147. Ncgc00016741-05
148. Ncgc00016741-06
149. Ncgc00016741-07
150. Ncgc00016741-09
151. Ncgc00022212-03
152. Ncgc00022212-04
153. Ncgc00022212-05
154. Sbi-0052674.p002
155. Ab00053176
156. Ft-0675241
157. Sw196447-4
158. T3058
159. Vu0239922-6
160. D01426
161. Tinidazole, Vetranal(tm), Analytical Standard
162. Ab00053176-16
163. Ab00053176_17
164. Ab00053176_18
165. A813669
166. Q1321320
167. Sr-01000000210-3
168. Sr-01000000210-5
169. Sr-01000000210-6
170. 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazol
171. Brd-k89125793-001-05-4
172. Z56763819
173. 1-(2'-(ethylsulfonyl)-ethyl]-2-methyl-5-nitroimidazole
174. Tinidazole, European Pharmacopoeia (ep) Reference Standard
175. Tinidazole, United States Pharmacopeia (usp) Reference Standard
176. Imidazole, 1-(2-(ethylsulfonyl)ethyl)-2-methyl-4-nitro-
177. Timtec-bb Sbb006917 Tinidazole 1-(2-(ethylsulfonyl)ethyl)-2-methyl-5-nitro-1h-imidazol
| Molecular Weight | 247.27 g/mol |
|---|---|
| Molecular Formula | C8H13N3O4S |
| XLogP3 | -0.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 247.06267708 g/mol |
| Monoisotopic Mass | 247.06267708 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 345 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Tindamax |
| PubMed Health | Tinidazole (By mouth) |
| Drug Classes | Amebicide, Extraintestinal, Amebicide, Intestinal, Antibiotic, Antiprotozoal |
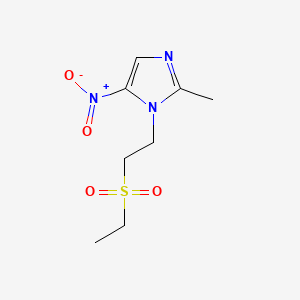
| Drug Label | Tinidazole is a synthetic antiprotozoal and antibacterial agent. It is 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazole, a second-generation 2-methyl-5-nitroimidazole, which has the following chemical structure:Tindamax pink oral tablets contain... |
| Active Ingredient | Tinidazole |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 250mg; 500mg |
| Market Status | Prescription |
| Company | Mission Pharma |
| 2 of 4 | |
|---|---|
| Drug Name | Tinidazole |
| PubMed Health | Tinidazole (By mouth) |
| Drug Classes | Amebicide, Extraintestinal, Amebicide, Intestinal, Antibiotic, Antiprotozoal |
| Drug Label | Tinidazole is a synthetic antiprotozoal and antibacterial agent. It is 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazole, a second-generation 2-methyl-5-nitroimidazole, which has the following chemical structure:Tinidazole tablets contain 250 mg o... |
| Active Ingredient | Tinidazole |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 500mg |
| Market Status | Prescription |
| Company | Unique Pharm Labs; Novel Labs; Roxane |
| 3 of 4 | |
|---|---|
| Drug Name | Tindamax |
| PubMed Health | Tinidazole (By mouth) |
| Drug Classes | Amebicide, Extraintestinal, Amebicide, Intestinal, Antibiotic, Antiprotozoal |
| Drug Label | Tinidazole is a synthetic antiprotozoal and antibacterial agent. It is 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazole, a second-generation 2-methyl-5-nitroimidazole, which has the following chemical structure:Tindamax pink oral tablets contain... |
| Active Ingredient | Tinidazole |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 250mg; 500mg |
| Market Status | Prescription |
| Company | Mission Pharma |
| 4 of 4 | |
|---|---|
| Drug Name | Tinidazole |
| PubMed Health | Tinidazole (By mouth) |
| Drug Classes | Amebicide, Extraintestinal, Amebicide, Intestinal, Antibiotic, Antiprotozoal |
| Drug Label | Tinidazole is a synthetic antiprotozoal and antibacterial agent. It is 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazole, a second-generation 2-methyl-5-nitroimidazole, which has the following chemical structure:Tinidazole tablets contain 250 mg o... |
| Active Ingredient | Tinidazole |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 500mg |
| Market Status | Prescription |
| Company | Unique Pharm Labs; Novel Labs; Roxane |
Antitrichomonal Agents
National Library of Medicine, SIS; ChemIDplus Record for Tinidazole (19387-91-8), MESH heading. Available from, as of March 15, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
MEDICATION: Antiprotozoal (Trichomonas, Giardia); antiamebic; antibacterial
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1685
Oral tinidazole is indicated in the treatment of amebic liver abscess caused by Entamoeba histolytica in both adults and pediatric patients older than three years of age. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2831
Oral tinidazole is indicated in the treatment of intestinal amebiasis caused by Entamoeba histolytica in both adults and pediatric patients older than three years of age. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2831
For more Therapeutic Uses (Complete) data for TINIDAZOLE (6 total), please visit the HSDB record page.
Tinidazole is distributed into breast milk at concentrations similar to those in serum. Because tinidazole can be detected in breast milk for up to 72 hours after administration, interruption of breast-feeding during tinidazole therapy and for three days following the last dose is recommended.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2832
FDA Pregnancy Risk Category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2832
Tinidazole crosses the placenta, and enters the fetal circulation. Therefore, it should not be administered to pregnant women during the first trimester.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2832
Adverse effects occurring in 1% or more of patients receiving tinidazole include GI effects (metallic/bitter taste, nausea, anorexia, dyspepsia/cramps/epigastric discomfort, vomiting, constipation) and nervous system effects (weakness/fatigue/malaise, dizziness, headache).
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 875
For more Drug Warnings (Complete) data for TINIDAZOLE (11 total), please visit the HSDB record page.
For the treatment of trichomoniasis caused by T. vaginalis in both female and male patients. Also for the treatment of giardiasis caused by G. duodenalis in both adults and pediatric patients older than three years of age and for the treatment of intestinal amebiasis and amebic liver abscess caused by E. histolytica in both adults and pediatric patients older than three years of age.
FDA Label
Tinidazole is a synthetic antiprotozoal agent. Tinidazole demonstrates activity both in vitro and in clinical infections against the following protozoa: Trichomonas vaginalis, Giardia duodenalis (also termed G. lamblia), and Entamoeba histolytica. Tinidazole does not appear to have activity against most strains of vaginal lactobacilli.
Antitrichomonal Agents
Agents used to treat trichomonas infections. (See all compounds classified as Antitrichomonal Agents.)
Alkylating Agents
Highly reactive chemicals that introduce alkyl radicals into biologically active molecules and thereby prevent their proper functioning. Many are used as antineoplastic agents, but most are very toxic, with carcinogenic, mutagenic, teratogenic, and immunosuppressant actions. They have also been used as components in poison gases. (See all compounds classified as Alkylating Agents.)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01XD02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01X - Other antibacterials
J01XD - Imidazole derivatives
J01XD02 - Tinidazole
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01A - Agents against amoebiasis and other protozoal diseases
P01AB - Nitroimidazole derivatives
P01AB02 - Tinidazole
Absorption
Rapidly and completely absorbed under fasting conditions. Administration with food results in a delay in Tmax of approximately 2 hours and a decline in Cmax of approximately 10% and an AUC of 901.6 ± 126.5 mcg hr/mL.
Route of Elimination
Tinidazole crosses the placental barrier and is secreted in breast milk. Tinidazole is excreted by the liver and the kidneys. Tinidazole is excreted in the urine mainly as unchanged drug (approximately 20-25% of the administered dose). Approximately 12% of the drug is excreted in the feces.
Volume of Distribution
50 L
Tinidazole is distributed into virtually all tissues and body fluids. Tinidazole also crosses the blood-brain barrier, placental barrier and is distributed into breast milk. Volume of distribution (VolD): about 50 L.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2831
Under fasted conditions tinidazole is rapidly and completely absorbed. Administration with food resulted in a delay in Tmax of approximately 2 hours and a decline in Cmax of approximately 10% and an AUC of 901.6 + or - 126.5 ug hr/mL.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2831
Time to peak concentration: 1.6 (+ or - 0.7 hours)
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2831
Elimination: Renal: 20 to 25% as unchanged drug. Fecal: 12%. In hemodialysis: 43% eliminated during 6 hour hemodialysis session.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2831
For more Absorption, Distribution and Excretion (Complete) data for TINIDAZOLE (6 total), please visit the HSDB record page.
Hepatic, mainly via CYP3A4. Tinidazole, like metronidazole, is significantly metabolized in humans prior to excretion. Tinidazole is partly metabolized by oxidation, hydroxylation and conjugation. Tinidazole is the major drug-related constituent in plasma after human treatment, along with a small amount of the 2-hydroxymethyl metabolite.
Tinidazole, like metronidazole, is significantly metabolized in humans prior to excretion. Tinidazole is partly metabolized by oxidation, hydroxylation and conjugation. Tinidazole is the major drug-related constituent in plasma after human treatment, along with a small amount of the 2-hydroxymethyl metabolite. Tinidazole is biotransformed mainly by CYP3A4. In an in vitro metabolic drug interaction study, tinidazole concentrations of up to 75 ug/mL did not inhibit the enzyme activities of CYP1A2, CYP2B6, CYP2C9, CYP2D6, CYP2E1 and CYP3A4.
Physicians Desk Reference 60th ed, Thomson PDR, Montvale, NJ 2006., p. 2670
The elimination half-life is 13.21.4 hours and the plasma half-life is 12 to 14 hours.
Elimination: 13.2 (+ or - 1.4) hours. Plasma: approximately 12 to 14 hours.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2831
Tinidazole is a prodrug and antiprotozoal agent. The nitro group of tinidazole is reduced in Trichomonas by a ferredoxin-mediated electron transport system. The free nitro radical generated as a result of this reduction is believed to be responsible for the antiprotozoal activity. It is suggested that the toxic free radicals covalently bind to DNA, causing DNA damage and leading to cell death. The mechanism by which tinidazole exhibits activity against Giardia and Entamoeba species is not known, though it is probably similar.
The nitro group of tinidazole is reduced by cell extracts of Trichomonas. As a result of this reduction a free nitro radical is generated which may be responsible for the antiprotozoal activity. The mechanism by which tinidazole exhibits activity against Giardia and Entamoeba species is not known.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2831
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.

GDUFA
DMF Review : Reviewed
Rev. Date : 2015-09-02
Pay. Date : 2015-08-18
DMF Number : 18824
Submission : 2005-09-26
Status : Active
Type : II

Certificate Number : R1-CEP 2006-143 - Rev 02
Issue Date : 2020-05-05
Type : Chemical
Substance Number : 1051
Status : Valid

NDC Package Code : 14799-2005
Start Marketing Date : 2010-08-16
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 6226
Submission : 1985-09-06
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 6544
Submission : 1986-08-19
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 1704
Submission : 1971-04-20
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 6275
Submission : 1986-04-21
Status : Inactive
Type : II


Registration Number : 221MF10180
Registrant's Address : Mahendra Industrial Estate, Ground floor, Plot no: 109-D, Road no: 29, Sion (E), Mumbai-400 022 India
Initial Date of Registration : 2009-08-07
Latest Date of Registration :

Date of Issue : 2022-08-10
Valid Till : 2025-08-05
Written Confirmation Number : WC-0219
Address of the Firm :

Date of Issue : 2019-09-03
Valid Till : 2022-09-02
Written Confirmation Number : WC-251
Address of the Firm :

Date of Issue : 2019-09-03
Valid Till : 2022-09-02
Written Confirmation Number : WC-0251
Address of the Firm :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Mission CDMO team brings reliability, resourcefulness, & responsiveness in executing your development project.
Mission CDMO team brings reliability, resourcefulness, & responsiveness in executing your development project.
RLD : Yes
TE Code : AB
Brand Name : TINDAMAX
Dosage Form : TABLET;ORAL
Dosage Strength : 250MG
Approval Date : 2004-05-17
Application Number : 21618
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AB
 Mission CDMO team brings reliability, resourcefulness, & responsiveness in executing your development project.
Mission CDMO team brings reliability, resourcefulness, & responsiveness in executing your development project.
RLD : Yes
TE Code : AB
Brand Name : TINDAMAX
Dosage Form : TABLET;ORAL
Dosage Strength : 500MG
Approval Date : 2004-05-17
Application Number : 21618
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AB
 Mission CDMO team brings reliability, resourcefulness, & responsiveness in executing your development project.
Mission CDMO team brings reliability, resourcefulness, & responsiveness in executing your development project.
RLD :
TE Code :
Brand Name : TINDAMAX
Dosage Form : TABLET; ORAL
Dosage Strength : 250MG
Approval Date :
Application Number : 21681
RX/OTC/DISCN :
RLD :
TE Code :
 Mission CDMO team brings reliability, resourcefulness, & responsiveness in executing your development project.
Mission CDMO team brings reliability, resourcefulness, & responsiveness in executing your development project.
RLD :
TE Code :
Brand Name : TINDAMAX
Dosage Form : TABLET; ORAL
Dosage Strength : 500MG
Approval Date :
Application Number : 21681
RX/OTC/DISCN :
RLD :
TE Code :
 Mission CDMO team brings reliability, resourcefulness, & responsiveness in executing your development project.
Mission CDMO team brings reliability, resourcefulness, & responsiveness in executing your development project.
RLD :
TE Code :
Brand Name : TINDAMAX
Dosage Form : TABLET; ORAL
Dosage Strength : 250MG
Approval Date :
Application Number : 21682
RX/OTC/DISCN :
RLD :
TE Code :
 Mission CDMO team brings reliability, resourcefulness, & responsiveness in executing your development project.
Mission CDMO team brings reliability, resourcefulness, & responsiveness in executing your development project.
RLD :
TE Code :
Brand Name : TINDAMAX
Dosage Form : TABLET; ORAL
Dosage Strength : 500MG
Approval Date :
Application Number : 21682
RX/OTC/DISCN :
RLD :
TE Code :
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : TINIDAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 250MG
Approval Date : 2015-08-04
Application Number : 203808
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : TINIDAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 500MG
Approval Date : 2015-08-04
Application Number : 203808
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : TINIDAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 250MG
Approval Date : 2012-04-30
Application Number : 201172
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : TINIDAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 250MG
Approval Date : 2013-10-09
Application Number : 202489
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Tronase
Dosage Form : Tinidazolo 500Mg 8 Joined' Oral Use
Dosage Strength : 8 CPR 500 mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Fasigyn
Dosage Form : FILM COATED PILL
Dosage Strength : 500 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Fasigin N
Dosage Form :
Dosage Strength : 14 Ov Vag 150 Mg + 22 Mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
24
PharmaCompass offers a list of Tinidazole API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Tinidazole manufacturer or Tinidazole supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Tinidazole manufacturer or Tinidazole supplier.
PharmaCompass also assists you with knowing the Tinidazole API Price utilized in the formulation of products. Tinidazole API Price is not always fixed or binding as the Tinidazole Price is obtained through a variety of data sources. The Tinidazole Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tinidazole manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tinidazole, including repackagers and relabelers. The FDA regulates Tinidazole manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tinidazole API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Tinidazole manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Tinidazole supplier is an individual or a company that provides Tinidazole active pharmaceutical ingredient (API) or Tinidazole finished formulations upon request. The Tinidazole suppliers may include Tinidazole API manufacturers, exporters, distributors and traders.
click here to find a list of Tinidazole suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Tinidazole DMF (Drug Master File) is a document detailing the whole manufacturing process of Tinidazole active pharmaceutical ingredient (API) in detail. Different forms of Tinidazole DMFs exist exist since differing nations have different regulations, such as Tinidazole USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Tinidazole DMF submitted to regulatory agencies in the US is known as a USDMF. Tinidazole USDMF includes data on Tinidazole's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Tinidazole USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Tinidazole suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Tinidazole Drug Master File in Japan (Tinidazole JDMF) empowers Tinidazole API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Tinidazole JDMF during the approval evaluation for pharmaceutical products. At the time of Tinidazole JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Tinidazole suppliers with JDMF on PharmaCompass.
A Tinidazole CEP of the European Pharmacopoeia monograph is often referred to as a Tinidazole Certificate of Suitability (COS). The purpose of a Tinidazole CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Tinidazole EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Tinidazole to their clients by showing that a Tinidazole CEP has been issued for it. The manufacturer submits a Tinidazole CEP (COS) as part of the market authorization procedure, and it takes on the role of a Tinidazole CEP holder for the record. Additionally, the data presented in the Tinidazole CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Tinidazole DMF.
A Tinidazole CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Tinidazole CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Tinidazole suppliers with CEP (COS) on PharmaCompass.
A Tinidazole written confirmation (Tinidazole WC) is an official document issued by a regulatory agency to a Tinidazole manufacturer, verifying that the manufacturing facility of a Tinidazole active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Tinidazole APIs or Tinidazole finished pharmaceutical products to another nation, regulatory agencies frequently require a Tinidazole WC (written confirmation) as part of the regulatory process.
click here to find a list of Tinidazole suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Tinidazole as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Tinidazole API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Tinidazole as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Tinidazole and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Tinidazole NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Tinidazole suppliers with NDC on PharmaCompass.
Tinidazole Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tinidazole GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tinidazole GMP manufacturer or Tinidazole GMP API supplier for your needs.
A Tinidazole CoA (Certificate of Analysis) is a formal document that attests to Tinidazole's compliance with Tinidazole specifications and serves as a tool for batch-level quality control.
Tinidazole CoA mostly includes findings from lab analyses of a specific batch. For each Tinidazole CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tinidazole may be tested according to a variety of international standards, such as European Pharmacopoeia (Tinidazole EP), Tinidazole JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tinidazole USP).
