
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
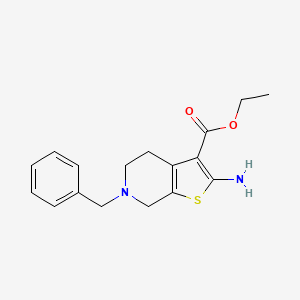

1. 2-amino-6-benzyl-4,5,6,7-tetrahydrothieno(2,3-c)-pyridine-3-carboxylic Acid Ethyl Ester
2. Nonflamin
3. Nonflamine
4. Tinoridin
5. Tinoridine Hydrochloride
6. Tinoridine Monohydrochloride
7. Y 3642
8. Y-3642
1. 24237-54-5
2. Ethyl 2-amino-6-benzyl-4,5,6,7-tetrahydrothieno[2,3-c]pyridine-3-carboxylate
3. Nonflamin (free Base)
4. Tinoridine [inn]
5. Y 3642
6. Y-3642
7. Nsc 158555
8. C9z9icz7yr
9. Nsc-158555
10. Ethyl 2-amino-6-benzyl-5,7-dihydro-4h-thieno[2,3-c]pyridine-3-carboxylate
11. Chembl592943
12. Dtxsid9023677
13. Tinoridine (inn)
14. 2-amino-3-ethoxycarbonyl-6-benzyl-4,5,6,7-tetrahydrothieno(2,3-c)pyridine
15. Ethyl 2-amino-6-benzyl-4,5,6,7-tetrahydrothieno(2,3-c)pyridine-3-carboxylate
16. Ethyl 2-amino-6-benzyl-4,5,6,7-tetrahydrothieno-[2,3-c]pyridine-3-carboxylate
17. 2-amino-6-benzyl-4,5,6,7-tetrahydrothieno(2,3-c)pyridine-3-carboxylic Acid Ethyl Ester
18. Ncgc00159451-02
19. Ethyl 2-amino-6-benzyl-4,5,6,7-tetrahydrothieno [2,3-c]pyridine-3-carboxylate
20. Thieno(2,3-c)pyridine-3-carboxylic Acid, 2-amino-6-benzyl-4,5,6,7-tetrahydro-, Ethyl Ester
21. Thieno[2,3-c]pyridine-3-carboxylic Acid, 2-amino-4,5,6,7-tetrahydro-6-(phenylmethyl)-, Ethyl Ester
22. Dtxcid403677
23. Tinoridino
24. Tinoridinum
25. Tinoridinum [inn-latin]
26. Tinoridino [inn-spanish]
27. 2-amino-6-benzyl-4,5,6,7-tetrahydrothieno[2,3-c]pyridine-3-carboxylic Acid Ethyl Ester
28. Ethyl 2-amino-6-benzyl-4,5,6,7-tetrahydrothieno[2,3-c]pyridine-3-carboxylate (tinoridine)
29. Thieno(2,3-c)pyridine-3-carboxylic Acid, 2-amino-4,5,6,7-tetrahydro-6-(phenylmethyl)-, Ethyl Ester
30. Cas-24237-54-5
31. Einecs 246-102-0
32. Unii-c9z9icz7yr
33. Brn 1082341
34. Tinoridine [mi]
35. Maybridge2_000001
36. Cbmicro_018439
37. 2-amino-3-aethoxycarbonyl-6-benzyl-4,5,6,7-tetrahydrothieno(2,3-c)pyridin
38. 2-amino-3-aethoxycarbonyl-6-benzyl-4,5,6,7-tetrahydrothieno(2,3-c)pyridin [german]
39. Tinoridine [who-dd]
40. Oprea1_041651
41. Oprea1_798781
42. Schembl24761
43. Mls004774001
44. Chebi:135353
45. Pfenfdgyvlafbr-uhfffaoysa-n
46. Hms1303a01
47. Bcp12825
48. Ccg-6706
49. Tox21_111678
50. Bdbm50304470
51. Mfcd00401417
52. Nsc158555
53. Akos000267028
54. Tox21_111678_1
55. Db13001
56. Hy-w032848
57. 2-amino-6-benzyl-4,5,6,7-tetrahydro-thieno[2,3-c]pyridine-3-carboxylic Acid Ethyl Ester
58. Idi1_001041
59. Thieno(2,3-c)pyridine-3-carboxylic Acid, 4,5,6,7-tetrahydro-2-amino-6-benzyl-, Ethyl Ester
60. Ncgc00159451-03
61. Ncgc00159451-04
62. Ncgc00159451-05
63. Ncgc00159451-06
64. Ac-27457
65. As-35595
66. Smr000103196
67. Bim-0018431.p001
68. Cs-0076872
69. Ft-0645518
70. Ns00027593
71. En300-03920
72. D08602
73. J-015409
74. J-524972
75. Sr-01000644920-1
76. Q27275368
77. Z56176137
78. F1673-2635
79. Thieno[2, 2-amino-6-benzyl-4,5,6,7-tetrahydro-, Ethyl Ester
80. Ethyl 2-amino-6-benzyl-4h,5h,6h,7h-thieno[2,3-c]pyridine-3-carboxylate
81. Thieno[2, 2-amino-4,5,6,7-tetrahydro-6-(phenylmethyl)-, Ethyl Ester
82. Ethyl 2-amino-6-(phenylmethyl)-4,5,6,7-tetrahydrothieno[2,3-c]pyridine-3-carboxylate
83. Ethyl 2-amino-6-benzyl-5, 7-dihydro-4h-thieno[2, 3-c]pyridine-3-carboxylate
| Molecular Weight | 316.4 g/mol |
|---|---|
| Molecular Formula | C17H20N2O2S |
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 83.8 |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 387 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
ABOUT THIS PAGE
