
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Klisyri
2. Kx-01
3. Kx2-391
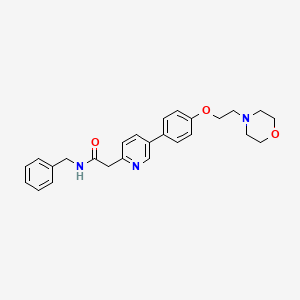
4. N-benzyl-2-(5-(4-(2-(4-morpholinyl)ethoxy)phenyl)-2-pyridinyl)acetamide
5. Tirbanibulin
1. Kx2-391
2. 897016-82-9
3. Tirbanibulin
4. Kx-01
5. Klisyri
6. Kx-2-391
7. Kx-2391
8. Kx01
9. Tirbanibulin [usan]
10. Kx2391
11. Chembl571546
12. 4v9848rs5g
13. Kx2-391;kx-01
14. 2-pyridineacetamide, 5-(4-(2-(4-morpholinyl)ethoxy)phenyl)-n-(phenylmethyl)-
15. N-benzyl-2-[5-[4-(2-morpholin-4-ylethoxy)phenyl]pyridin-2-yl]acetamide
16. 2-[5-[4-(2-morpholin-4-ylethoxy)phenyl]pyridin-2-yl]-~{n}-(phenylmethyl)ethanamide
17. Unii-4v9848rs5g
18. N-benzyl-2-(5-(4-(2-morpholin-4-ylethoxy)phenyl)pyridin-2-yl)acetamide
19. Kx 01
20. Tirbanibulin (usan/inn)
21. Tirbanibulin [inn]
22. Kx2-391 (tirbanibulin)
23. Tirbanibulin (kx2-391)
24. Mls006011272
25. Schembl153779
26. Tirbanibulin [who-dd]
27. Gtpl7957
28. Dtxsid30237862
29. Hms3656j15
30. Hms3673e15
31. Tirbanibulin [orange Book]
32. Bcp02845
33. Ex-a2434
34. Xkb01682
35. Bdbm50303801
36. Nsc756643
37. Nsc800779
38. S2700
39. Who 10864
40. Zinc43152787
41. N-benzyl-2-(5-{4-[2-(morpholin-4-yl)ethoxy]phenyl}pyridin-2-yl)acetamide
42. Bcp9000828
43. Ccg-264983
44. Cs-0248
45. Db06137
46. Kx2-391; Kx-01
47. Nsc-756643
48. Nsc-800779
49. Sb16619
50. Ncgc00346644-01
51. Ncgc00346644-05
52. Ac-35458
53. As-73245
54. Hy-10340
55. Kx 2-391
56. Smr004703022
57. Db-119272
58. Sw219670-1
59. C77028
60. D11691
61. A915990
62. Brd-k29968218-001-01-6
63. Q27888424
64. 2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)-n-benzylacetamide
65. 2-(5-(4-(2-morpholinoethoxyl)phenyl)pyridin-2-yl)-n-benzylacetamide
66. 2-[5-[4-(2-morpholin-4-ylethoxy)phenyl]pyridin-2-yl]-n-(phenylmethyl)acetamide
67. 2-pyridineacetamide,5-[4-[2-(4-morpholinyl)ethoxy]phenyl]-n-(phenylmethyl)-
68. 5-[4-[2-(4-morpholinyl)ethoxy]phenyl]-n-(phenylmethyl)-2-pyridineacetamide
69. Dn0
| Molecular Weight | 431.5 g/mol |
|---|---|
| Molecular Formula | C26H29N3O3 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 9 |
| Exact Mass | 431.22089180 g/mol |
| Monoisotopic Mass | 431.22089180 g/mol |
| Topological Polar Surface Area | 63.7 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 540 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Tirbanibulin is indicated for the topical treatment of actinic keratosis on the face or scalp.
Klisyri is indicated for the field treatment of non-hyperkeratotic, non-hypertrophic actinic keratosis (Olsen grade 1) of the face or scalp in adults.
In clinical trials composed comprising patients with actinic keratosis of the face or scalp, tirbanibulin promoted complete clearance of actinic keratosis lesions at day 57 in treated areas in 44-54% of patients compared to 5-13% of patients who received the placebo. Actinic keratosis is a chronic, pre-malignant condition characterized by lesions and proliferation of neoplastic keratinocytes. Tirbanibulin mediates an anti-proliferative effect by inhibiting tubulin polymerization and Src kinase signalling. Tirbanibulin inhibited primary tumour growth and metastasis in many preclinical animal models of cancer. In human triple-negative breast cancer, or estrogen receptor (ER)/progesterone receptor (PR)/human epidermal growth factor receptor 2 (HER2)-negative tumour, xenografts, tirbanibulin suppressed tumour growth and metastasis. Tirbanibulin was also shown to restore functional ER expression in ER-negative breast tumours. Tirbanibulin promoted synergistic tumour growth inhibition of breast cancer cell lines when used in combination with tamoxifen and paclitaxel. In a clinical trial comprising patients with advanced solid tumours, dose-limiting toxicities of tirbanibulin included elevated liver transaminases, neutropenia and fatigue.
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
D06BX03
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06B - Chemotherapeutics for topical use
D06BX - Other chemotherapeutics
D06BX03 - Tirbanibulin
Absorption
Tirbanibulin demonstrates good oral bioavailability. Following topical administration of doses ranging from 54 to 295 mg on the face or scalp, the steady-state concentration of tirbanibulin was achieved by 72 hours. At five days following initial administration, the mean Cmax was 0.340.30 ng/mL in subjects who received topical treatment on the face and 0.180.10 ng/mL in subjects who received topical treatment on the scalp. The mean AUC24 was 5.03.9 h x ng/mL in subjects who received topical treatment on the face and 3.21.9 h x ng/mL in subjects who received topical treatment on the scalp. The median Tmax was about seven hours.
Route of Elimination
There is limited information on the route of elimination of tirbanibulin.
Volume of Distribution
There is limited information on the volume of distribution of tirbanibulin. In mouse HT29 xenograft studies, the tissue to plasma ration of tirbanibulin was 1.52.
Clearance
There is limited information on the clearance rate of tirbanibulin.
_In vitro_, tirbanibulin is mainly metabolized by CYP3A4, and to a lesser extent, CYP2C8. In adult subjects with actinic keratosis, detected metabolites were KX2-5036 and KX2-5163, which were pharmacologically inactive metabolites with the highest plasma concentrations of 0.09 ng/mL and 0.12 ng/mL, respectively.
The half-life is about 4 hours.
Src tyrosine kinases regulate normal cell growth: the expression of Src kinase is upregulated during the normal hair cycle during the proliferative anagen phase. Additionally, Src tyrosine kinases act as key modulators of cancer cell proliferation, survival, angiogenesis, migration, invasion and metastasis. Src is frequently upregulated in various epithelial tumours including colon, breast and pancreas compared with the adjacent normal tissues. The expression and activity of Src are also enhanced in human actinic keratosis, which is characterized by hyperproliferative premalignant skin lesions. The pathogenesis of actinic keratosis commonly involves skin inflammation, oxidative stress, immunosuppression, impaired apoptosis, mutagenesis, dysregulation of keratinocyte growth and proliferation, and tissue remodelling. _In vitro_ studies suggest that Src plays a predominant role in the early stages of human skin tumour development, rather than at later stages of tumour progression. The exact mechanism of tirbanibulin as a topical treatment of actinic keratosis has not been fully elucidated; however, it mainly works by inhibiting fast proliferating cells. Tirbanibulin is a non-ATP competitive Src kinase inhibitor and tubulin polymerization inhibitor. It binds to the peptide substrate binding site of Src, a primary target of tirbanibulin, and blocking its downstream signalling pathways that promote cancer cell migration, proliferation, and survival. Tublin is responsible for cell migration, protein transport, and mitosis: tibranibulin directly binds to the colchicine-binding site of beta-tubulin and causes induces tubulin depolymerization. It is also hypothesized that inhibition of Src can also contribute to the inhibitory effects on microtubule polymerization. At low nanomolar concentrations, tirbanibulin induces G2/M phase cell cycle arrest in a reversible and dose-dependent manner. By inhibiting microtubule polymerization, tirbanibulin also induces mitotic catastrophe.
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
46
PharmaCompass offers a list of Tirbanibulin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Tirbanibulin manufacturer or Tirbanibulin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Tirbanibulin manufacturer or Tirbanibulin supplier.
PharmaCompass also assists you with knowing the Tirbanibulin API Price utilized in the formulation of products. Tirbanibulin API Price is not always fixed or binding as the Tirbanibulin Price is obtained through a variety of data sources. The Tirbanibulin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tirbanibulin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tirbanibulin, including repackagers and relabelers. The FDA regulates Tirbanibulin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tirbanibulin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Tirbanibulin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Tirbanibulin supplier is an individual or a company that provides Tirbanibulin active pharmaceutical ingredient (API) or Tirbanibulin finished formulations upon request. The Tirbanibulin suppliers may include Tirbanibulin API manufacturers, exporters, distributors and traders.
click here to find a list of Tirbanibulin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Tirbanibulin DMF (Drug Master File) is a document detailing the whole manufacturing process of Tirbanibulin active pharmaceutical ingredient (API) in detail. Different forms of Tirbanibulin DMFs exist exist since differing nations have different regulations, such as Tirbanibulin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Tirbanibulin DMF submitted to regulatory agencies in the US is known as a USDMF. Tirbanibulin USDMF includes data on Tirbanibulin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Tirbanibulin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Tirbanibulin suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Tirbanibulin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Tirbanibulin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Tirbanibulin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Tirbanibulin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Tirbanibulin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Tirbanibulin suppliers with NDC on PharmaCompass.
Tirbanibulin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tirbanibulin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tirbanibulin GMP manufacturer or Tirbanibulin GMP API supplier for your needs.
A Tirbanibulin CoA (Certificate of Analysis) is a formal document that attests to Tirbanibulin's compliance with Tirbanibulin specifications and serves as a tool for batch-level quality control.
Tirbanibulin CoA mostly includes findings from lab analyses of a specific batch. For each Tirbanibulin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tirbanibulin may be tested according to a variety of international standards, such as European Pharmacopoeia (Tirbanibulin EP), Tirbanibulin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tirbanibulin USP).
