
Synopsis
Synopsis
0
VMF
0
Australia

Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
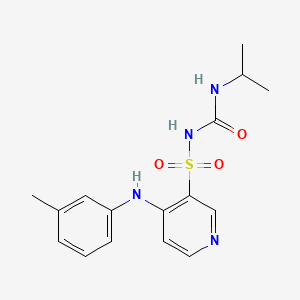

1. 1-isopropyl-3-((4-(3-methylphenylamino)pyridine)-3-sulfonyl)urea
2. 1-isopropyl-3-((4-m-toluidino-3-pyridyl)sulfonyl)urea
3. Demadex
4. Torasemide
1. Torasemide
2. 56211-40-6
3. Demadex
4. Luprac
5. Torasemida
6. Torasemidum
7. Torasemidum [inn-latin]
8. Torasemida [inn-spanish]
9. Unat
10. Ac-4464
11. Torasemide Anhydrous
12. Jdl 464
13. Torem
14. Bm-02015
15. Ac4464
16. N-(isopropylcarbamoyl)-4-(m-tolylamino)pyridine-3-sulfonamide
17. Soaanz
18. Upcard
19. 1-isopropyl-3-((4-m-toluidino-3-pyridyl)sulfonyl)urea
20. Ac 4464
21. Jdl-464
22. Bm 02015
23. 1-[4-(3-methylanilino)pyridin-3-yl]sulfonyl-3-propan-2-ylurea
24. Torasemide [inn]
25. Torsemide (demadex)
26. Torasemide, Anhydrous
27. Bm02.015
28. N-(((1-methylethyl)amino)carbonyl)-4-((3-methylphenyl)amino)-3-pyridinesulfonamide
29. Bm-02.015
30. Mls001165687
31. Chebi:9637
32. W31x2h97fb
33. 3-pyridinesulfonamide, N-(((1-methylethyl)amino)carbonyl)-4-((3-methylphenyl)amino)-
34. Torsemide [usan]
35. Ncgc00016879-01
36. Smr000466313
37. Toradiur
38. Cas-56211-40-6
39. 1-{4-[(3-methylphenyl)amino]pyridine-3-sulfonyl}-3-(propan-2-yl)urea
40. Dsstox_cid_3690
41. N-[(isopropylamino)carbonyl]-4-[(3-methylphenyl)amino]pyridine-3-sulfonamide
42. 3-pyridinesulfonamide, N-[[(1-methylethyl)amino]carbonyl]-4-[(3-methylphenyl)amino]-
43. Dsstox_rid_77149
44. Dsstox_gsid_23690
45. Dilutol
46. Torocard
47. Sutril
48. Torrem
49. Torasemide N
50. 4-[(3-methylphenyl)amino]-n-(propan-2-ylcarbamoyl)pyridine-3-sulfonamide
51. N-{[(1-methylethyl)amino]carbonyl}-4-[(3-methylphenyl)amino]pyridine-3-sulfonamide
52. Torsemide (usp)
53. Demadex (tn)
54. Luprac (tn)
55. Ccris 6736
56. Torasemide (jan/inn)
57. Torsemide [usan:usp]
58. Gj-1090
59. Brn 0498515
60. Unii-w31x2h97fb
61. Mfcd00866166
62. Ks-1123
63. Pw-2132
64. Spectrum_001776
65. Torsemide [mi]
66. Torasemide [jan]
67. Prestwick0_001030
68. Prestwick1_001030
69. Prestwick2_001030
70. Prestwick3_001030
71. Spectrum2_001142
72. Spectrum3_001832
73. Spectrum4_000290
74. Spectrum5_001699
75. Torsemide [vandf]
76. Torasemide [mart.]
77. Torsemide [usp-rs]
78. Chembl1148
79. Torasemide [who-dd]
80. Schembl41184
81. Bspbio_001219
82. Bspbio_003503
83. Kbiogr_000820
84. Kbioss_002257
85. Cid_41781
86. Mls000759418
87. Mls001195611
88. Mls001424121
89. Mls006010744
90. Bidd:gt0623
91. Spectrum1505211
92. Spbio_001063
93. Spbio_003080
94. Bpbio1_001341
95. Gtpl7312
96. Zinc5823
97. Torsemide [orange Book]
98. Dtxsid2023690
99. Torsemide [usp Impurity]
100. Bdbm64107
101. Kbio2_002256
102. Kbio2_004824
103. Kbio2_007392
104. Kbio3_003008
105. Torasemide For System Suitability
106. Torasemide [ep Monograph]
107. Torsemide [usp Monograph]
108. Hms1571m21
109. Hms1922n05
110. Hms2051l21
111. Hms2098m21
112. Hms2234n14
113. Hms3373g20
114. Hms3393l21
115. Hms3715m21
116. Hms3744g19
117. Albb-027267
118. Bcp07286
119. Hy-b0247
120. Tox21_110662
121. Ccg-40257
122. S1698
123. Stl388026
124. Torsemide, >=98% (hplc), Solid
125. Akos015894937
126. Tox21_110662_1
127. Bm02015
128. Db00214
129. Nc00238
130. Ncgc00016879-02
131. Ncgc00016879-03
132. Ncgc00016879-04
133. Ncgc00016879-06
134. Ncgc00016879-07
135. Ncgc00095141-01
136. Ncgc00095141-02
137. Ncgc00095141-03
138. Ac-18762
139. Ab00514011
140. Ft-0630689
141. Ft-0675300
142. T2538
143. D00382
144. T72621
145. Ab00514011-09
146. Ab00514011_10
147. Ab00514011_11
148. 211t406
149. A830961
150. Q419948
151. Sr-01000759362
152. Torasemide Anhydrous [ema Epar Veterinary]
153. Q-201846
154. Sr-01000759362-5
155. Brd-k30480208-001-05-2
156. Torsemide, United States Pharmacopeia (usp) Reference Standard
157. 1-isopropyl-3-((4-m-toluidino-3-pyridyl)sulphonyl)urea
158. 4-(3-methylanilino)-n-(propan-2-ylcarbamoyl)pyridine-3-sulfonamide
159. Torasemide Anhydrous, European Pharmacopoeia (ep) Reference Standard
160. 1-isopropyl-3-[[4-(3-methylanilino)-3-pyridyl]sulfonyl]urea;torsemide
161. N-(isopropylcarbamoyl)-4-[(3-methylphenyl)amino]pyridine-3-sulfonamide
162. N-[(propan-2-yl)carbamoyl]-4-[(3-methylphenyl)amino]pyridine-3-sulfonamide
163. N-({4-[(3-methylphenyl)imino]-1,4-dihydropyridin-3-yl}sulfonyl)propane-2-carbamimidic Acid
164. Torasemide For System Suitability, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 348.4 g/mol |
|---|---|
| Molecular Formula | C16H20N4O3S |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 348.12561169 g/mol |
| Monoisotopic Mass | 348.12561169 g/mol |
| Topological Polar Surface Area | 109 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 518 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Demadex |
| PubMed Health | Torsemide |
| Drug Classes | Cardiovascular Agent |
| Drug Label | DEMADEX (torsemide) is a diuretic of the pyridine-sulfonylurea class. Its chemical name is 1-isopropyl-3-[(4-m-toluidino-3-pyridyl) sulfonyl] urea and its structural formula is:Its empirical formula is C16H20N4O3S, its pKa is 7.1, and its molecular... |
| Active Ingredient | Torsemide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 100mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Meda Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Torsemide |
| PubMed Health | Torsemide |
| Drug Classes | Cardiovascular Agent |
| Drug Label | DEMADEX (torsemide) is a diuretic of the pyridine-sulfonylurea class. Its chemical name is 1-isopropyl-3-[(4-m-toluidino-3-pyridyl) sulfonyl] urea and its structural formula is:Its empirical formula is C16H20N4O3S, its pKa is 7.1, and its molecular... |
| Active Ingredient | Torsemide |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 50mg/5ml (10mg/ml); 5mg; 20mg/2ml (10mg/ml); 100mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Teva; Apotex; Hetero Labs Ltd Iii; Pliva Pharm Ind; Sun Pharm Inds; Aurobindo Pharma; Par Pharm; Roxane; Luitpold; Eurohlth Intl |
| 3 of 4 | |
|---|---|
| Drug Name | Demadex |
| PubMed Health | Torsemide |
| Drug Classes | Cardiovascular Agent |
| Drug Label | DEMADEX (torsemide) is a diuretic of the pyridine-sulfonylurea class. Its chemical name is 1-isopropyl-3-[(4-m-toluidino-3-pyridyl) sulfonyl] urea and its structural formula is:Its empirical formula is C16H20N4O3S, its pKa is 7.1, and its molecular... |
| Active Ingredient | Torsemide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 100mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Meda Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Torsemide |
| PubMed Health | Torsemide |
| Drug Classes | Cardiovascular Agent |
| Drug Label | DEMADEX (torsemide) is a diuretic of the pyridine-sulfonylurea class. Its chemical name is 1-isopropyl-3-[(4-m-toluidino-3-pyridyl) sulfonyl] urea and its structural formula is:Its empirical formula is C16H20N4O3S, its pKa is 7.1, and its molecular... |
| Active Ingredient | Torsemide |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 50mg/5ml (10mg/ml); 5mg; 20mg/2ml (10mg/ml); 100mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Teva; Apotex; Hetero Labs Ltd Iii; Pliva Pharm Ind; Sun Pharm Inds; Aurobindo Pharma; Par Pharm; Roxane; Luitpold; Eurohlth Intl |
Torasemide is indicated for the treatment of edema associated with congestive heart failure, renal or hepatic diseases. From this condition, it has been observed that torasemide is very effective in cases of kidney failure. As well, torasemide is approved to be used as an antihypertensive agent either alone or in combination with other antihypertensives.
FDA Label
For treatment of clinical signs, including oedema and effusion, related to congestive heart failure in dogs.
It is widely known that administration of torasemide can attenuate renal injury and reduce the severity of acute renal failure. This effect is obtained by increasing urine output and hence, facilitating fluid, acid-base and potassium control. This effect is obtained by the increase in the excretion of urinary sodium and chloride. Several reports have indicated that torasemide presents a long-lasting diuresis and less potassium excretion which can be explained by the effect that torasemide has on the renin-angiotensin-aldosterone system. This effect is very similar to the effect observed with the administration of combination therapy with [furosemide] and [spironolactone] and it is characterized by a decrease in plasma brain natriuretic peptide and improved measurements of left ventricular function. Above the aforementioned effect, torasemide presents a dual effect .in which the inhibition of aldosterone which donates torasemide with a potassium-sparing action. Torasemide has been shown to reduce extracellular fluid volume and blood pressure in hypertensive patients suffering from chronic kidney disease. As well, some reports have indicated that torasemide can reduce myocardial fibrosis by reducing the collagen accumulation. This effect is suggested to be related to the decrease in aldosterone which in order has been shown to reduce the production of the enzyme procollagen type I carboxy-terminal proteinase which is known to be overexpressed in heart failure patients.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Sodium Potassium Chloride Symporter Inhibitors
Agents that inhibit SODIUM-POTASSIUM-CHLORIDE SYMPORTERS which are concentrated in the thick ascending limb at the junction of the LOOP OF HENLE and KIDNEY TUBULES, DISTAL. They act as DIURETICS. Excess use is associated with HYPOKALEMIA and HYPERGLYCEMIA. (See all compounds classified as Sodium Potassium Chloride Symporter Inhibitors.)
Diuretics
Agents that promote the excretion of urine through their effects on kidney function. (See all compounds classified as Diuretics.)
QC03CA04
C03CA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C03 - Diuretics
C03C - High-ceiling diuretics
C03CA - Sulfonamides, plain
C03CA04 - Torasemide
Absorption
Torasemide is the diuretic with the highest oral bioavailability even in advanced stages of chronic kidney disease. This bioavailability tends to be higher than 80% regardless of the patient condition. The maximal serum concentration is reported to be of 1 hour and the absorption parameters are not affected by its use concomitantly with food.
Route of Elimination
Torasemide is mainly hepatically processed and excreted in the feces from which about 70-80% of the administered dose is excreted by this pathway. On the other hand, about 20-30% of the administered dose is found in the urine.
Volume of Distribution
The volume of distribution of torasemide is 0.2 L/kg.
Clearance
The clearance rate of torasemide is considerably reduced by the presence of renal disorders.
Torasemide is extensively metabolized in the liver and only 20% of the dose remains unchanged and it is recovered in the urine. Metabolized via the hepatic CYP2C8 and CYP2C9 mainly by reactions of hydroxylation, oxidation and reduction to 5 metabolites. The major metabolite, M5, is pharmacologically inactive. There are 2 minor metabolites, M1, possessing one-tenth the activity of torasemide, and M3, equal in activity to torasemide. Overall, torasemide appears to account for 80% of the total diuretic activity, while metabolites M1 and M3 account for 9% and 11%, respectively.
Torasemide has known human metabolites that include N-[(4-{[3-(hydroxymethyl)phenyl]imino}-1,4-dihydropyridin-3-yl)sulfonyl]propane-2-carbamimidic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The average half-life of torasemide is 3.5 hours.
As mentioned above, torasemide is part of the loop diuretics and thus, it acts by reducing the oxygen demand in the medullary thick ascending loop of Henle by inhibiting the Na+/K+/Cl- pump on the luminal cell membrane surface. This action is obtained by the binding of torasemide to a chloride ion-binding site of the transport molecule. Torasemide is known to have an effect in the renin-angiotensin-aldosterone system by inhibiting the downstream cascade after the activation of angiotensin II. This inhibition will produce a secondary effect marked by the reduction of the expression of aldosterone synthase, TGF-B1 and thromboxane A2 and a reduction on the aldosterone receptor binding.
 Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15222
Submission : 2001-01-03
Status : Inactive
Type : II
 JPN Pharma offers excellence in API manufacturing through precision, innovation & quality, delivering solutions to the pharma industry
JPN Pharma offers excellence in API manufacturing through precision, innovation & quality, delivering solutions to the pharma industry
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15738
Submission : 2001-11-26
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21576
Submission : 2008-04-29
Status : Active
Type : II
Certificate Number : R0-CEP 2007-174 - Rev 02
Issue Date : 2011-08-03
Type : Chemical
Substance Number : 2132
Status : Expired


GDUFA
DMF Review : Reviewed
Rev. Date : 2014-01-06
Pay. Date : 2013-09-10
DMF Number : 27406
Submission : 2013-08-02
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2022-02-18
Pay. Date : 2022-01-13
DMF Number : 36664
Submission : 2022-01-20
Status : Active
Type : II

NDC Package Code : 72166-010
Start Marketing Date : 2020-08-08
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15222
Submission : 2001-01-03
Status : Inactive
Type : II
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15738
Submission : 2001-11-26
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18466
Submission : 2005-06-29
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 19237
Submission : 2006-03-08
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17489
Submission : 2004-06-21
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21954
Submission : 2008-10-01
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15562
Submission : 2001-07-31
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15563
Submission : 2001-07-31
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2018-01-19
Pay. Date : 2017-12-21
DMF Number : 18360
Submission : 2007-06-26
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16851
Submission : 2003-09-17
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
About the Company : Interquim, founded in 1978, is now part of the Ferrer HealthTech division. Interquim specializes in the development of competitive processes for manufacturing high added-value APIs...
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for the pharmaceutical and biotech industries. LGM is also a full service CDMO providing formulation, ...
About the Company : Jai Radhe Sales was founded in 1999 as an out-of-the-box distribution firm specializing in the global supply of high-quality pharmaceutical ingredients. The firm provides complete ...
About the Company : HRV Global is a leading global manufacturer, seller & exporter of a wide range of APIs, advanced intermediates, pellets, food grade chemicals, food additives & food ingredients. It...
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
About the Company : Founded in 1935, TAPI Technology & API Services has a long-standing tradition of advancing health through innovation and dedication. Today, we proudly build upon this legacy, drivi...
 JPN Pharma offers excellence in API manufacturing through precision, innovation & quality, delivering solutions to the pharma industry
JPN Pharma offers excellence in API manufacturing through precision, innovation & quality, delivering solutions to the pharma industry
About the Company : JPN Pharma is a premier pharmaceutical company in India, specializing in the development and manufacturing of Active Pharmaceutical Ingredients (APIs) and drug intermediates. Headq...
About the Company : Founded in 1986 by Mr. P.V. Ramaprasad Reddy, Mr. K. Nityananda Reddy and a small group of highly committed professionals, Aurobindo Pharma was born off a vision. The company comme...

About the Company : Guangzhou Tosun Pharmaceutical was founded in 1999, which mainly focuses on importation & exportation of Active Pharmaceutical Ingrediants, Chemical Raw Materials, Intermediate, Ex...

About the Company : Ipca Laboratories, headquartered in Mumbai, India, is a prominent pharmaceutical enterprise established in 1949. Over the years, Ipca has emerged as a leading player in India's pha...

About the Company : Prudence Pharmachem, founded in 2003, operates in the production and advancement of a diverse array of premium pharmaceutical bulk drugs (API) and their advanced intermediates. The...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
71
PharmaCompass offers a list of Torsemide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Torsemide manufacturer or Torsemide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Torsemide manufacturer or Torsemide supplier.
PharmaCompass also assists you with knowing the Torsemide API Price utilized in the formulation of products. Torsemide API Price is not always fixed or binding as the Torsemide Price is obtained through a variety of data sources. The Torsemide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Torsemide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Torsemide, including repackagers and relabelers. The FDA regulates Torsemide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Torsemide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Torsemide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Torsemide supplier is an individual or a company that provides Torsemide active pharmaceutical ingredient (API) or Torsemide finished formulations upon request. The Torsemide suppliers may include Torsemide API manufacturers, exporters, distributors and traders.
click here to find a list of Torsemide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Torsemide DMF (Drug Master File) is a document detailing the whole manufacturing process of Torsemide active pharmaceutical ingredient (API) in detail. Different forms of Torsemide DMFs exist exist since differing nations have different regulations, such as Torsemide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Torsemide DMF submitted to regulatory agencies in the US is known as a USDMF. Torsemide USDMF includes data on Torsemide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Torsemide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Torsemide suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Torsemide Drug Master File in Japan (Torsemide JDMF) empowers Torsemide API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Torsemide JDMF during the approval evaluation for pharmaceutical products. At the time of Torsemide JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Torsemide suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Torsemide Drug Master File in Korea (Torsemide KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Torsemide. The MFDS reviews the Torsemide KDMF as part of the drug registration process and uses the information provided in the Torsemide KDMF to evaluate the safety and efficacy of the drug.
After submitting a Torsemide KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Torsemide API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Torsemide suppliers with KDMF on PharmaCompass.
A Torsemide CEP of the European Pharmacopoeia monograph is often referred to as a Torsemide Certificate of Suitability (COS). The purpose of a Torsemide CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Torsemide EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Torsemide to their clients by showing that a Torsemide CEP has been issued for it. The manufacturer submits a Torsemide CEP (COS) as part of the market authorization procedure, and it takes on the role of a Torsemide CEP holder for the record. Additionally, the data presented in the Torsemide CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Torsemide DMF.
A Torsemide CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Torsemide CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Torsemide suppliers with CEP (COS) on PharmaCompass.
A Torsemide written confirmation (Torsemide WC) is an official document issued by a regulatory agency to a Torsemide manufacturer, verifying that the manufacturing facility of a Torsemide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Torsemide APIs or Torsemide finished pharmaceutical products to another nation, regulatory agencies frequently require a Torsemide WC (written confirmation) as part of the regulatory process.
click here to find a list of Torsemide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Torsemide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Torsemide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Torsemide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Torsemide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Torsemide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Torsemide suppliers with NDC on PharmaCompass.
Torsemide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Torsemide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Torsemide GMP manufacturer or Torsemide GMP API supplier for your needs.
A Torsemide CoA (Certificate of Analysis) is a formal document that attests to Torsemide's compliance with Torsemide specifications and serves as a tool for batch-level quality control.
Torsemide CoA mostly includes findings from lab analyses of a specific batch. For each Torsemide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Torsemide may be tested according to a variety of international standards, such as European Pharmacopoeia (Torsemide EP), Torsemide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Torsemide USP).
