
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
FDF
0
Listed Dossiers
0
EDQM
0
USP
0
JP
0
Others
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Gsk 1120212
2. Gsk-1120212
3. Gsk1120212
4. Jtp 74057
5. Jtp-74057
6. Jtp74057
1. 871700-17-3
2. Gsk1120212
3. Mekinist
4. Gsk-1120212
5. Jtp 74057
6. Jtp-74057
7. Gsk 1120212
8. Trametinib (gsk1120212)
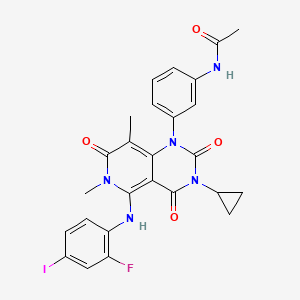
9. N-[3-[3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl-2,4,7-trioxopyrido[4,3-d]pyrimidin-1(2h)-yl]phenyl]acetamide
10. Gsk212
11. Tmt212
12. Trametinib [usan]
13. Chebi:75998
14. Tmt-212
15. 33e86k87qn
16. Trametinib (usan)
17. N-(3-{3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2h)-yl}phenyl)acetamide
18. N-[3-[3-cyclopropyl-5-(2-fluoro-4-iodoanilino)-6,8-dimethyl-2,4,7-trioxopyrido[4,3-d]pyrimidin-1-yl]phenyl]acetamide
19. Acetamide, N-(3-(3-cyclopropyl-5-((2-fluoro-4-iodophenyl)amino)-3,4,6,7-tetrahydro-6,8- Dimethyl-2,4,7-trioxopyrido(4,3-d)pyrimidin-1(2h)-yl)phenyl)-
20. N-(3-(3-cyclopropyl-5-((2-fluoro-4-iodophenyl)amino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2h)-yl)phenyl)acetamide
21. Unii-33e86k87qn
22. Trametinib [usan:inn]
23. Trametinibum
24. Jtp74057
25. N-(3-(3-cyclopropyl-5-((2-fluoro-4-iodophenyl)amino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7- Tetrahydropyrido(4,3-d)pyrimidin-1(2h)-yl)phenyl)acetamide
26. N-(3-(3-cyclopropyl-5-((2-fluoro-4-iodophenyl)amino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido(4,3-d)pyrimidin-1(2h)-yl)phenyl)acetamide
27. N-(3-{3-cyclopropyl-5-((2-fluoro-4-iodophenyl)amino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7- Tetrahydropyrido(4,3-d)pyrimidin-1(2h)-yl}phenyl)acetamide
28. Qom
29. Trametinib [mi]
30. Trametinib (gsk1120212jtp 74057)
31. Trametinib [inn]
32. Trametinib [vandf]
33. Trametinib [who-dd]
34. Schembl170938
35. Gtpl6495
36. Gsk1120212 (trametinib)
37. Chembl2103875
38. Ex-a022
39. Bcpp000218
40. Dtxsid901007381
41. Hms3295i05
42. Hms3656j11
43. Bcp02307
44. Bdbm50531540
45. Mfcd17215075
46. Nsc758246
47. Nsc800956
48. S2673
49. Zinc43100709
50. Akos015850628
51. Am90271
52. Ccg-264976
53. Cs-0060
54. Db08911
55. Ex-5957
56. Nsc-758246
57. Nsc-800956
58. Sb16553
59. Ncgc00263180-01
60. Ncgc00263180-07
61. Ncgc00263180-14
62. Ac-25891
63. As-19382
64. Hy-10999
65. N-[3-[3-cyclopropyl-5-(2-fluoro-4-iodo-anilino)-6,8-dimethyl-2,4,7-trioxo-pyrido[4,3-d]pyrimidin-1-yl]phenyl]acetamide
66. Ft-0688438
67. Sw218089-2
68. A25168
69. D10175
70. Gsk1120212 - Jtp-74057
71. Gsk1120212,jtp-74057, Gsk212
72. Sr-01000941589
73. A1-01871
74. J-523325
75. Q7833138
76. Sr-01000941589-1
77. Brd-k12343256-001-01-4
78. Acetamide, N-[3-[3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl-2,4,7-trioxopyrido[4,3-d]pyrimidin-1(2h)-yl]phenyl]-
79. N-(3-(3-cyclopropyl-5-(2-fluoro-4-iodophenylamino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2h)-yl)phenyl)acetamide
80. N-[3-[3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl-2,4,7-trioxopyrido[4,3-d]pyrimidin-1(2h)-yl]phe Nyl]acetamide
81. N-[3-[3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-6,8-dimethyl-2,4,7-trioxopyrido[3,4-e]pyrimidin-1-yl]phenyl]acetamide
82. N-{3-[3-cyclopropyl-5-(2-fluoro-4-iodoanilino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2h)-yl]phenyl}ethanimidic Acid
83. N-{3-[3-cyclopropyl-5-(2-fluoro-4-iodophenylamino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydro-2h-pyrido[4,3-d]pyrimidin-1-yl]phenyl}acetamide
| Molecular Weight | 615.4 g/mol |
|---|---|
| Molecular Formula | C26H23FIN5O4 |
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 615.07788 g/mol |
| Monoisotopic Mass | 615.07788 g/mol |
| Topological Polar Surface Area | 102 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 1090 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Mekinist |
| PubMed Health | Trametinib (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Trametinib dimethyl sulfoxide is a kinase inhibitor. The chemical name is acetamide, N-[3-[3-cyclopropyl-5-[(2-fluoro-4- iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl- 2,4,7-trioxopyrido[4,3-d]pyrimidin-1(2H)-yl]phenyl]-, compound with 1,1-sul... |
| Active Ingredient | Trametinib dimethyl sulfoxide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 1mg non-solvated parent; eq 0.5mg non-solvated parent; eq 2mg non-solvated parent |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 2 of 2 | |
|---|---|
| Drug Name | Mekinist |
| PubMed Health | Trametinib (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Trametinib dimethyl sulfoxide is a kinase inhibitor. The chemical name is acetamide, N-[3-[3-cyclopropyl-5-[(2-fluoro-4- iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl- 2,4,7-trioxopyrido[4,3-d]pyrimidin-1(2H)-yl]phenyl]-, compound with 1,1-sul... |
| Active Ingredient | Trametinib dimethyl sulfoxide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 1mg non-solvated parent; eq 0.5mg non-solvated parent; eq 2mg non-solvated parent |
| Market Status | Prescription |
| Company | Glaxosmithkline |
Trametinib is indicated for the treatment of unresectable or metastatic melanoma with BRAF V600E or V600K mutations, as detected by an FDA-approved test [FDA]. In May 2018, it was approved for use with [DB08912] for the treatment of treat anaplastic thyroid cancer caused by an abnormal BRAF V600E gene.
FDA Label
* Melanoma:
Trametinib as monotherapy or in combination with dabrafenib is indicated for the treatment of adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation (see sections 4. 4 and 5. 1).
Trametinib monotherapy has not demonstrated clinical activity in patients who have progressed on a prior BRAF inhibitor therapy (see section 5. 1).
* Adjuvant treatment of melanoma:
Trametinib in combination with dabrafenib is indicated for the adjuvant treatment of adult patients with Stage III melanoma with a BRAF V600 mutation, following complete resection.
* Non-small cell lung cancer (NSCLC):
Trametinib in combination with dabrafenib is indicated for the treatment of adult patients with advanced non-small cell lung cancer with a BRAF V600 mutation.
Trametinib is an anticancer agent which causes apoptosis (or programmed cell death) and inhibits cell proliferation, which are both important in the treatment of malignancies.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L01EE01
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EE - Mitogen-activated protein kinase (mek) inhibitors
L01EE01 - Trametinib
Absorption
Trametinib is readily absorbed. When an oral administration of trametinib was given to patients with BRAF V600 mutation-positive melanoma, peak plasma concentration occurred 1.5 hours post-dose (Tmax). A single 2 mg oral dose has a bioavailability of 72%. When a dose of 2mg/day is given, the peak plasma concentration (Cmax) is 22.2 ng/mL.
Route of Elimination
80% of the dose is excreted in the feces. <20% of the dose is excreted in the urine with <0.1% of the excreted dose in the form of the parent compound.
Volume of Distribution
Apparent volume of distribution (Vd/F) = 214 L
Clearance
Apparent clearance = 4.9 L/h
Trametinib is metabolized predominantly via deacetylation alone or with mono-oxygenation or in combination with glucuronidation biotransformation pathways in vitro. Deacetylation is likely mediated by hydrolytic enzymes, such as carboxyl-esterases or amidases. The cytochrome P450 enzyme system is not involved with the metabolism of trametinib. The predominant circulating component in the plasma is the parent drug.
Elimination half-life = 3.9-4.8 days.
Trametinib is a reversible, allosteric inhibitor of mitogen-activated extracellular signal regulated kinase 1 _(MEK1)_ and _MEK2_ activation and of_ MEK1_ and _MEK2_ kinase activity. MEK proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway, which promotes cellular proliferation. Trametinib helps with melanoma with the BRAF V600E or V600K as the mutation results in the constitutive activation of the BRAF pathway which includes MEK1 and MEK2.
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..
Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40493
Submission : 2024-09-29
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2022-10-18
Pay. Date : 2022-09-12
DMF Number : 37504
Submission : 2022-09-13
Status : Active
Type : II

NDC Package Code : 59651-710
Start Marketing Date : 2024-01-11
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (50kg/50kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 31589
Submission : 2017-03-31
Status : Active
Type : II

Date of Issue : 2022-09-02
Valid Till : 2025-05-05
Written Confirmation Number : WC-0349
Address of the Firm :

NDC Package Code : 54893-0062
Start Marketing Date : 2017-03-31
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2022-03-31
Pay. Date : 2022-01-06
DMF Number : 35525
Submission : 2020-12-31
Status : Active
Type : II

NDC Package Code : 54893-0062
Start Marketing Date : 2017-03-31
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT



USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39561
Submission : 2024-06-19
Status : Active
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
ERAS-254 (naporafenib) , an oral Pan-Raf inhibitor is being investigated with trametinib (MEK inhibitor) in patients with NRAS-mutant (NRASm) melanoma.
Lead Product(s): Naporafenib,Trametinib
Therapeutic Area: Oncology Brand Name: ERAS-254
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 18, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Naporafenib,Trametinib
Therapeutic Area : Oncology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Erasca Starts Phase 3 Trial for Naporafenib in Melanoma
Details : ERAS-254 (naporafenib) , an oral Pan-Raf inhibitor is being investigated with trametinib (MEK inhibitor) in patients with NRAS-mutant (NRASm) melanoma.
Brand Name : ERAS-254
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 18, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The net proceeds will be used to advance the clinical development of ERAS-254 (naporafenib), which is being evaluated in late-stage clinical trials for the treatment of RAS Q61X-mutated melanoma.
Lead Product(s): Naporafenib,Trametinib
Therapeutic Area: Oncology Brand Name: ERAS-254
Study Phase: Phase IProduct Type: Small molecule
Sponsor: J.P. Morgan
Deal Size: $184.0 million Upfront Cash: Undisclosed
Deal Type: Public Offering May 21, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Naporafenib,Trametinib
Therapeutic Area : Oncology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : J.P. Morgan
Deal Size : $184.0 million
Deal Type : Public Offering
Erasca Announces Closing of Underwritten Offering of Common Stock and Additional Shares
Details : The net proceeds will be used to advance the clinical development of ERAS-254 (naporafenib), which is being evaluated in late-stage clinical trials for the treatment of RAS Q61X-mutated melanoma.
Brand Name : ERAS-254
Molecule Type : Small molecule
Upfront Cash : Undisclosed
May 21, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Erasca will use the proceeds to fund R&D of its product candidates, including ERAS-254 (naporafenib) with trametinib for patients with RAS Q61X solid tumors, and other developmental programs.
Lead Product(s): Naporafenib,Trametinib
Therapeutic Area: Oncology Brand Name: ERAS-254
Study Phase: Phase IProduct Type: Small molecule
Sponsor: BofA Securities
Deal Size: $45.0 million Upfront Cash: Undisclosed
Deal Type: Private Placement March 27, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Naporafenib,Trametinib
Therapeutic Area : Oncology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : BofA Securities
Deal Size : $45.0 million
Deal Type : Private Placement
Erasca Announces $45 Million Oversubscribed Private Placement Financing
Details : Erasca will use the proceeds to fund R&D of its product candidates, including ERAS-254 (naporafenib) with trametinib for patients with RAS Q61X solid tumors, and other developmental programs.
Brand Name : ERAS-254
Molecule Type : Small molecule
Upfront Cash : Undisclosed
March 27, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The collaboration will support the clinical development of the pan-RAF inhibitor ERAS-254 (naporafenib) in combination with trametinib for the treatment of patients with RAS Q61X solid tumors.
Lead Product(s): Naporafenib,Trametinib
Therapeutic Area: Oncology Brand Name: ERAS-254
Study Phase: Phase IProduct Type: Small molecule
Sponsor: Novartis Pharmaceuticals Corporation
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Collaboration February 14, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Naporafenib,Trametinib
Therapeutic Area : Oncology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Novartis Pharmaceuticals Corporation
Deal Size : Undisclosed
Deal Type : Collaboration
Erasca Announces Clinical Trial Collaboration and Supply Agreements for Trametinib
Details : The collaboration will support the clinical development of the pan-RAF inhibitor ERAS-254 (naporafenib) in combination with trametinib for the treatment of patients with RAS Q61X solid tumors.
Brand Name : ERAS-254
Molecule Type : Small molecule
Upfront Cash : Undisclosed
February 14, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
LXH254 (naporafenib) is a potent and selective pan-RAF inhibitor, it is under phase 3 clinical development in combination with Mekinist (trametinib) for the treatment of NRAS-mutated metastatic melanoma .
Lead Product(s): Naporafenib,Trametinib
Therapeutic Area: Oncology Brand Name: ERAS-254
Study Phase: Phase IProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 11, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Naporafenib,Trametinib
Therapeutic Area : Oncology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : LXH254 (naporafenib) is a potent and selective pan-RAF inhibitor, it is under phase 3 clinical development in combination with Mekinist (trametinib) for the treatment of NRAS-mutated metastatic melanoma .
Brand Name : ERAS-254
Molecule Type : Small molecule
Upfront Cash : Not Applicable
December 11, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
ERAS-254 (naporafenib) is a potent and selective pan-RAF inhibitor, with a potential first-in-class and best-in-class profile, which is being investigated in combination with MEK inhibitor trametinib (MEKINIST®) in patients with RAS Q61X solid tumors.
Lead Product(s): Naporafenib,Trametinib
Therapeutic Area: Oncology Brand Name: ERAS-254
Study Phase: Phase IProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 29, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Naporafenib,Trametinib
Therapeutic Area : Oncology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : ERAS-254 (naporafenib) is a potent and selective pan-RAF inhibitor, with a potential first-in-class and best-in-class profile, which is being investigated in combination with MEK inhibitor trametinib (MEKINIST®) in patients with RAS Q61X solid tumors.
Brand Name : ERAS-254
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 29, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Tafinlar is a combination of dabrafenib mesylate and trametinib that targets two different kinases in the RAS/RAF/MEK/ERK pathway. This combination resulted in greater growth inhibition of BRAF V600 mutation-positive tumor cell lines.
Lead Product(s): Dabrafenib Mesylate,Trametinib
Therapeutic Area: Oncology Brand Name: Tafinlar
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 16, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Dabrafenib Mesylate,Trametinib
Therapeutic Area : Oncology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Tafinlar is a combination of dabrafenib mesylate and trametinib that targets two different kinases in the RAS/RAF/MEK/ERK pathway. This combination resulted in greater growth inhibition of BRAF V600 mutation-positive tumor cell lines.
Brand Name : Tafinlar
Molecule Type : Small molecule
Upfront Cash : Not Applicable
March 16, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
CFT1946 is an orally bioavailable BiDAC™ degrader designed to be potent and selective against BRAF V600 mutant targets. C4T is advancing CFT1946 to the clinic to study treatment for BRAF V600 mutant solid tumors including NSCLC, colorectal cancer, and melanoma.
Lead Product(s): CFT1946,Trametinib
Therapeutic Area: Oncology Brand Name: CFT1946
Study Phase: Phase I/ Phase IIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable January 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : CFT1946,Trametinib
Therapeutic Area : Oncology
Highest Development Status : Phase I/ Phase II
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : CFT1946 is an orally bioavailable BiDAC™ degrader designed to be potent and selective against BRAF V600 mutant targets. C4T is advancing CFT1946 to the clinic to study treatment for BRAF V600 mutant solid tumors including NSCLC, colorectal cancer, and ...
Brand Name : CFT1946
Molecule Type : Small molecule
Upfront Cash : Not Applicable
January 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
DCC-3116 is orally administered, potent, and highly selective switch-control inhibitor designed to inhibit cancer autophagy, key tumor survival mechanism in cancer cells, by inhibiting ULK1/2 kinases, which have been shown to be enzymes responsible for initiating autophagy.
Lead Product(s): DCC-3116,Trametinib
Therapeutic Area: Oncology Brand Name: DCC-3116
Study Phase: Phase I/ Phase IIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 10, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : DCC-3116,Trametinib
Therapeutic Area : Oncology
Highest Development Status : Phase I/ Phase II
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : DCC-3116 is orally administered, potent, and highly selective switch-control inhibitor designed to inhibit cancer autophagy, key tumor survival mechanism in cancer cells, by inhibiting ULK1/2 kinases, which have been shown to be enzymes responsible for i...
Brand Name : DCC-3116
Molecule Type : Small molecule
Upfront Cash : Not Applicable
September 10, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
SNR1611 (trametinib) was the first drug candidate identified at Genuv with the ATRIVIEW® platform. It showed the most potent neurogenerative and neuroprotective effects in a library of FDA-approved drug compounds.
Lead Product(s): Trametinib
Therapeutic Area: Neurology Brand Name: SNR1611
Study Phase: IND EnablingProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 18, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Trametinib
Therapeutic Area : Neurology
Highest Development Status : IND Enabling
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Publication in Nature Molecular Psychiatry Supports Genuv’s Alternate Theory for Treatment of Al...
Details : SNR1611 (trametinib) was the first drug candidate identified at Genuv with the ATRIVIEW® platform. It showed the most potent neurogenerative and neuroprotective effects in a library of FDA-approved drug compounds.
Brand Name : SNR1611
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 18, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Spexotras
Dosage Form : Powder for oral solution, solution
Dosage Strength : 0.05 mg/ml
Packaging : Bottle 1item
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Mekinist
Dosage Form : Film-Coated Tablets
Dosage Strength : 0.5mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Mekinist
Dosage Form : Film-Coated Tablets
Dosage Strength : 0.5mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Mekinist
Dosage Form : Film-Coated Tablets
Dosage Strength : 2mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Mekinist
Dosage Form : Film-Coated Tablets
Dosage Strength : 2mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : MEKINIST
Dosage Form : TABLET
Dosage Strength : 0.5MG
Packaging : 30
Approval Date :
Application Number : 2409623
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : MEKINIST
Dosage Form : TABLET
Dosage Strength : 2MG
Packaging : 30
Approval Date :
Application Number : 2409658
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : MEKINIST
Dosage Form : POWDER FOR SOLUTION
Dosage Strength : 4.7MG
Packaging :
Approval Date :
Application Number : 2539993
Regulatory Info : Prescription
Registration Country : Canada

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Regulatory Info :
Registration Country : Australia
Brand Name : Mekinist
Dosage Form :
Dosage Strength :
Packaging : 30
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Australia
Brand Name : Mekinist
Dosage Form :
Dosage Strength :
Packaging : 30
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Australia
Brand Name : Mekinist
Dosage Form :
Dosage Strength :
Packaging : 30
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Australia
Brand Name : Mekinist
Dosage Form :
Dosage Strength :
Packaging : 30
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Australia
Brand Name : Mekinist
Dosage Form :
Dosage Strength :
Packaging : 30
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Australia
Brand Name : Mekinist
Dosage Form :
Dosage Strength :
Packaging : 30
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Regulatory Info : Originator
Registration Country : South Africa
Brand Name : MEKINIST 2 mg
Dosage Form : FCT
Dosage Strength : 2mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : MEQSEL 0,50 mg
Dosage Form : FCT
Dosage Strength : 0.5mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
Patent Expiration Date : 2027-11-29
US Patent Number : 7378423*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 204114
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2027-11-29

Patent Expiration Date : 2032-07-28
US Patent Number : 9399021*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 204114
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2032-07-28

Patent Expiration Date : 2031-04-15
US Patent Number : 8952018*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 204114
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2031-04-15

Patent Expiration Date : 2032-07-28
US Patent Number : 9155706*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 204114
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2032-07-28

Patent Expiration Date : 2031-04-15
US Patent Number : 8952018*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 204114
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2031-04-15

Patent Expiration Date : 2030-10-15
US Patent Number : 8703781
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 204114
Patent Use Code : U-2302
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2030-10-15

Patent Expiration Date : 2025-06-10
US Patent Number : 8835443
Drug Substance Claim :
Drug Product Claim :
Application Number : 204114
Patent Use Code : U-1581
Delist Requested :
Patent Use Description : IN COMBINATION WITH DA...
Patent Expiration Date : 2025-06-10

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : ODE-182
Exclusivity Expiration Date : 2025-04-30
Application Number : 204114
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : ODE-183
Exclusivity Expiration Date : 2025-05-04
Application Number : 204114
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : ODE-148
Exclusivity Expiration Date : 2024-06-22
Application Number : 204114
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : I-908
Exclusivity Expiration Date : 2026-03-16
Application Number : 204114
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : ODE-428
Exclusivity Expiration Date : 2030-03-16
Application Number : 204114
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : I-895
Exclusivity Expiration Date : 2025-06-22
Application Number : 204114
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : ODE-182
Exclusivity Expiration Date : 2025-04-30
Application Number : 204114
Product Number : 2
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : ODE-183
Exclusivity Expiration Date : 2025-05-04
Application Number : 204114
Product Number : 2
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : ODE-148
Exclusivity Expiration Date : 2024-06-22
Application Number : 204114
Product Number : 2
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : I-908
Exclusivity Expiration Date : 2026-03-16
Application Number : 204114
Product Number : 2
Exclusivity Details :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Patent Expiration Date : 2025-06-10
Date Granted : 2011-04-05
Brand Name : MEKINIST
Patent Number : 2569850
Filing Date : 2005-06-10
Strength per Unit : 0.5 mg
Dosage Form : Tablet
Human Or VET : Human
Route of Administration : Oral
Patent Expiration Date : 2025-06-10
Date Granted : 2011-04-05

Patent Expiration Date : 2025-06-10
Date Granted : 2011-04-05
Brand Name : MEKINIST
Patent Number : 2569850
Filing Date : 2005-06-10
Strength per Unit : 1.0 mg
Dosage Form : Tablet
Human Or VET : Human
Route of Administration : Oral
Patent Expiration Date : 2025-06-10
Date Granted : 2011-04-05

Patent Expiration Date : 2025-06-10
Date Granted : 2011-04-05
Brand Name : MEKINIST
Patent Number : 2569850
Filing Date : 2005-06-10
Strength per Unit : 2.0 mg
Dosage Form : Tablet
Human Or VET : Human
Route of Administration : Oral
Patent Expiration Date : 2025-06-10
Date Granted : 2011-04-05

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?