
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
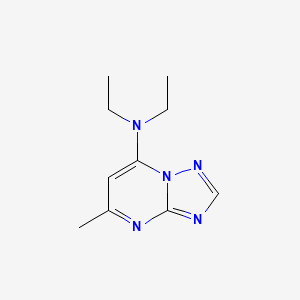

1. Rocornal
2. Trapymin
1. 15421-84-8
2. Rocornal
3. Trapymin
4. Avantrin
5. Trapymine
6. N,n-diethyl-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine
7. Ar 12008
8. Ar-12008
9. (1,2,4)triazolo(1,5-a)pyrimidin-7-amine, N,n-diethyl-5-methyl-
10. [1,2,4]triazolo[1,5-a]pyrimidin-7-amine,n,n-diethyl-5-methyl-
11. 7-diethylamino-5-methyl-s-triazolo(1,5-a)pyrimidine
12. Mls000567667
13. Eyg5y6355e
14. N,n-diethyl-5-methyl-(1,2,4)triazolo(1,5-a)pyrimidine-7-amine
15. Ncgc00016715-01
16. S-triazolo(1,5-a)pyrimidin-7-amine, N,n-diethyl-5-methyl-
17. Smr000154170
18. Cas-15421-84-8
19. Dsstox_cid_25416
20. Dsstox_rid_80865
21. Dsstox_gsid_45416
22. 7-(diethylamino)-5-methyl-s-triazolo(1,5-a)pyrimidine
23. Trapidilum
24. Trapidilum [inn-latin]
25. [1,2,4]triazolo[1,5-a]pyrimidin-7-amine, N,n-diethyl-5-methyl-
26. Einecs 239-434-2
27. Trapidil [inn:ban:jan]
28. Brn 0186842
29. Unii-eyg5y6355e
30. 7-(diethylamino)-5-methyl-s-triazolo[1,5-a]pyrimidine
31. Rocornal (tn)
32. Trapidil,(s)
33. Mfcd00193104
34. 5-methyl-7-diethylamino-s-triazolo-(1,5-a)-pyrimidine
35. Opera_id_461
36. 5-methyl-7-diaethylamino-s-triazolo(1.5-a)pyrimidin [german]
37. Trapidil [inn]
38. Trapidil [jan]
39. Trapidil [mi]
40. Prestwick0_001012
41. Prestwick1_001012
42. Prestwick2_001012
43. Prestwick3_001012
44. Trapidil (jp17/inn)
45. Trapidil [mart.]
46. Trapidil [who-dd]
47. Schembl33563
48. Bspbio_001163
49. Mls000881142
50. Spbio_003034
51. 5-methyl-7-diaethylamino-s-triazolo(1.5-a)pyrimidin
52. Bpbio1_001281
53. Chembl132767
54. Zinc2202
55. Trapidil [ep Monograph]
56. Trapidil, >=98% (hplc)
57. Dtxsid0045416
58. Chebi:32254
59. Hms1571k05
60. Hms1735k19
61. Hms2098k05
62. Hms2231g07
63. Hms3372j09
64. Hms3715k05
65. Bcp09350
66. Hy-b1016
67. Tox21_110578
68. S4736
69. Akos001093408
70. Tox21_110578_1
71. Ccg-221012
72. Cs-4529
73. Db09283
74. Hs-0048
75. N,n-diethyl-4-methyl-1,5,7,9-tetrazabicyclo[4.3.0]nona-2,4,6,8-tetraen-2-amine
76. Ncgc00016715-02
77. Ncgc00016715-03
78. Ncgc00016715-04
79. Ac-32565
80. Ab00457635
81. Ft-0763046
82. D01220
83. F31376
84. Ab00457635-12
85. 421t848
86. A927178
87. Sr-01000673280
88. Q2449982
89. Sr-01000673280-3
90. 5-methyl-7-diethylamino-s-triazolo(1,5-a)pyrimidine
91. Brd-k95763993-001-03-7
92. Z56791867
93. 5-methyl-7-(diethylamino)-s-triazolo[1,5-a]pyrimidine
94. 7-(diethylamino)-5-methyl-2-triazolo[1,5-a]pyrimidine
95. Trapidil, European Pharmacopoeia (ep) Reference Standard
96. 7-(diethylamino)-5-methyl-s-triazolo(1,5-a)pyrimidine.
97. Diethyl-(5-methyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-yl)amine
98. N,n-diethyl-5-methyl[1,2,4]triazolo[1,5-a]pyrimidin-7-amine #
99. N,n-diethyl-n-(5-methyl[1,2,4]triazolo[1,5-a]pyrimidin-7-yl)amine
| Molecular Weight | 205.26 g/mol |
|---|---|
| Molecular Formula | C10H15N5 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 205.13274550 g/mol |
| Monoisotopic Mass | 205.13274550 g/mol |
| Topological Polar Surface Area | 46.3 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 206 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used in the treatment of chronic stable angina.
Trapidil exerts vasodilatory and antiplatelet effects. It also inhibits the activity of platelet derived growth factor (PDGF).
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
Phosphodiesterase Inhibitors
Compounds which inhibit or antagonize the biosynthesis or actions of phosphodiesterases. (See all compounds classified as Phosphodiesterase Inhibitors.)
C - Cardiovascular system
C01 - Cardiac therapy
C01D - Vasodilators used in cardiac diseases
C01DX - Other vasodilators used in cardiac diseases
C01DX11 - Trapidil
Absorption
Trapidil has a Tmax of 1 h.
Clearance
The apparent clearance is 179 mL/min for a single dose and 273 mL/min for steady state dosing.
The half life of elimination is 1.31 h for a single dose and 1.14 h for steady state dosing.
Trapidil is thought to inhibit cyclic adenosine monophosphate (cAMP) phosphodiesterase enzymes. The resultant increase in cAMP potentiates the inhibition of platelets by adenosine. The reduction in platelet activation is likely responsible for the decrease in thromboxane A2 generation seen with trapidil. The increase in cAMP is also likely responsible for the vasdilatory action of trapidil. The increase in protein kinase A activity due to increased cAMP activated L-type calcium channels in the heart leading to increased depolarization and a positive inotropic effect. Lastly, PKA inactivates Raf-1, an activator of mitogen activated protein kinase (MAPK), which leads to a reduction in MAPK activation. This reduction in MAPK prevents mitogenesis due to PDGF binding to PDGF receptors.
Global Sales Information

Market Place

ABOUT THIS PAGE
28
PharmaCompass offers a list of Trapidil API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Trapidil manufacturer or Trapidil supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Trapidil manufacturer or Trapidil supplier.
PharmaCompass also assists you with knowing the Trapidil API Price utilized in the formulation of products. Trapidil API Price is not always fixed or binding as the Trapidil Price is obtained through a variety of data sources. The Trapidil Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Trapidil manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Trapidil, including repackagers and relabelers. The FDA regulates Trapidil manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Trapidil API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Trapidil supplier is an individual or a company that provides Trapidil active pharmaceutical ingredient (API) or Trapidil finished formulations upon request. The Trapidil suppliers may include Trapidil API manufacturers, exporters, distributors and traders.
click here to find a list of Trapidil suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Trapidil Drug Master File in Japan (Trapidil JDMF) empowers Trapidil API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Trapidil JDMF during the approval evaluation for pharmaceutical products. At the time of Trapidil JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Trapidil suppliers with JDMF on PharmaCompass.
Trapidil Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Trapidil GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Trapidil GMP manufacturer or Trapidil GMP API supplier for your needs.
A Trapidil CoA (Certificate of Analysis) is a formal document that attests to Trapidil's compliance with Trapidil specifications and serves as a tool for batch-level quality control.
Trapidil CoA mostly includes findings from lab analyses of a specific batch. For each Trapidil CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Trapidil may be tested according to a variety of international standards, such as European Pharmacopoeia (Trapidil EP), Trapidil JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Trapidil USP).
