
Synopsis
Synopsis
0
CEP/COS
0
NDC API
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
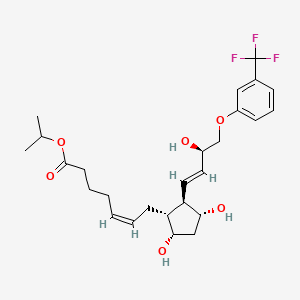

1. (((1r)-(1alpha(z),2beta(1e,3r*),3alpha,5alpha))-7-(3,5-dihydroxy-2-(3-hydroxy-4-(3-trifluoromethyl)phenoxy)-1-butenyl)cyclopentyl)-5-heptenoic Acid, 1-methylethyl Ester
2. Al 6221
3. Al-6221
4. Al6221
5. Travatan
6. Travatan Z
7. Z, Travatan
1. 157283-68-6
2. Travatan
3. Travatan Z
4. Izba
5. Al-6221
6. Travaprost
7. Trovoprost
8. Otx-tp
9. Chebi:746859
10. Propan-2-yl (z)-7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-[(e,3r)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]but-1-enyl]cyclopentyl]hept-5-enoate
11. Al6221
12. Wj68r08kx9
13. Nsc-760366
14. Travoprost [usan]
15. Travatanz
16. (z)-isopropyl 7-((1r,2r,3r,5s)-3,5-dihydroxy-2-((r,e)-3-hydroxy-4-(3-(trifluoromethyl)phenoxy)but-1-en-1-yl)cyclopentyl)hept-5-enoate
17. Isopropyl (z)-7-((1r,2r,3r,5s)-3,5-dihydroxy-2-{(1e,3r)-3-hydroxy-4-[(alpha,alpha,alpha-trifluoro-m-tolyl)oxy]-1-butenyl}cyclopentyl)-5-heptenoate
18. Travatan (tn)
19. 5-heptenoic Acid, 7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-[(1e,3r)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]cyclopentyl]-, 1-methylethyl Ester, (5z)-
20. Propan-2-yl (5z)-7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-[(1e,3r)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]but-1-en-1-yl]cyclopentyl]hept-5-enoate
21. Propan-2-yl (5z)-7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-{(1e,3r)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]but-1-en-1-yl}cyclopentyl]hept-5-enoate
22. Travoprostum
23. Unii-wj68r08kx9
24. (((1r)-(1alpha(z),2beta(1e,3r*),3alpha,5alpha))-7-(3,5-dihydroxy-2-(3-hydroxy-4-(3-trifluoromethyl)phenoxy)-1-butenyl)cyclopentyl)-5-heptenoic Acid, 1-methylethyl Ester
25. (+)-fluprostenol Isopropyl Ester
26. Travoprost [usan:usp:inn:ban]
27. Travoprost In Bulk
28. Travoprostintermediates
29. Travoprost [mi]
30. Travoprost [inn]
31. Travoprost [jan]
32. Travoprost [vandf]
33. Travoprost [mart.]
34. Travoprost [usp-rs]
35. Travoprost [who-dd]
36. Schembl93818
37. Travoprost (jan/usp/inn)
38. (1r-(1alpha(z),2beta(1e,3r*),3alpha,5alpha))-7-(3,5-dihydroxy-2-(3-hydroxy-4-(3-(trifluoromethyl)phenoxy)-1-butenyl)cyclopentyl)-5-heptenoic Acid, 1-methylethyl Ester
39. (z)-7-((1r,2r,3r,5s)-3,5-dihydroxy-2-((1e,3r)-3-hydroxy-4-((alpha,alpha,alpha-trifluoro-m-isopropyl-tolyl)oxy)-1-butenyl)cyclopentyl)-5-heptenoate
40. 5-heptenoic Acid, 7-(3,5-dihydroxy-2-(3-hydroxy-4-(3-(trifluoromethyl)phenoxy)-1-butenyl)cyclopentyl)-, 1-methylethyl Ester, (1r-(1alpha(z),2beta(1e,3r*),3alpha,5alpha))-
41. Travoprost [ema Epar]
42. Gtpl7102
43. Chembl1200799
44. Travoprost [orange Book]
45. Dtxsid80896948
46. Travoprost [usp Monograph]
47. Duotrav Component Travoprost
48. Ex-a1772
49. Hy-b0584
50. Zinc4474682
51. Bdbm50248302
52. S3738
53. Akos024458039
54. Travoprost Component Of Duotrav
55. Ac-6103
56. Am84515
57. Ccg-269692
58. Db00287
59. Nsc 760366
60. Ncgc00346741-02
61. 5-heptenoic Acid, 7-((1r,2r,3r,5s)-3,5-dihydroxy-2-((1e,3r)-3-hydroxy-4-(3-(trifluoromethyl)phenoxy)-1-butenyl)cyclopentyl)-, 1-methylethyl Ester, (5z)-
62. Bs-15509
63. Fluprostenol Isopropyl Ester;al6221;flu-ipr
64. D01964
65. 283t686
66. Sr-01000942266
67. Sr-01000946860
68. J-502633
69. Q2193376
70. Sr-01000942266-1
71. Sr-01000946860-1
72. Fluprostenol Isopropyl Ester, >=98%, Ethanol Solution
73. (1r-(1.alpha.(z),2.beta.(1e,3r*),3.alpha.,5.alpha.))-7-(3,5-dihydroxy-2-(3-hydroxy-4-(3-(trifluoromethyl)phenoxy)-1-butenyl)cyclopentyl)-5-heptenoic Acid, 1-methylethyl Ester
74. (5z)-7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-[(1e,3r)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]cyclopentyl]-5-heptenoic Acid 1-methyethyl Ester
75. (z)-isopropyl 7-((1r,2r)-3,5-dihydroxy-2-((s,e)-3-hydroxy-4-(3-(trifluoromethyl)phenoxy)but-1-en-1-yl)cyclopentyl)hept-5-enoate
76. (z)-isopropyl 7-((1r,2r,3r,5s)-3,5-dihydroxy-2-((r,e)-3-hydroxy-4-(3-(trifluoromethyl) Phenoxy)but-1-en-1-yl)cyclopentyl)hept-5-enoate
77. (z)-isopropyl7-((1r,2r,3r,5s)-3,5-dihydroxy-2-((r,e)-3-hydroxy-4-(3-(trifluoromethyl)phenoxy)but-1-en-1-yl)cyclopentyl)hept-5-enoate
78. Isopropyl (z)-7-((1r,2r,3r,5s)-3,5-dihydroxy-2-((1e,3r)-3-hydroxy-4-((.alpha.,.alpha.,.alpha.-trifluoro-m-tolyl)oxy)-1-butenyl)cyclopentyl)-5-heptenoate
79. Isopropyl (z)-7-((1r,2r,3r,5s)-3,5-dihydroxy-2-((3r,e)-3-hydroxy-4-(3-(trifluoromethyl)-phenoxy)-but-1-enyl)-cyclopentyl)-hept-5-enoate
| Molecular Weight | 500.5 g/mol |
|---|---|
| Molecular Formula | C26H35F3O6 |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 13 |
| Exact Mass | 500.23857332 g/mol |
| Monoisotopic Mass | 500.23857332 g/mol |
| Topological Polar Surface Area | 96.2 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 693 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Travatan z |
| PubMed Health | Travoprost (Into the eye) |
| Drug Classes | Antiglaucoma |
| Drug Label | Travoprost is a synthetic prostaglandin F analogue. Its chemical name is [1R-[1(Z),2(1E,3R*),3,5]]-7-[3,5-Dihydroxy-2-[3-hydroxy-4-[3-(trifluoromethyl) phenoxy]-1-butenyl]cyclopentyl]-5-heptenoic acid, 1-methylethylester. It has a molecular f... |
| Active Ingredient | Travoprost |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.004% |
| Market Status | Prescription |
| Company | Alcon Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Travoprost |
| PubMed Health | Travoprost (Into the eye) |
| Drug Classes | Antiglaucoma |
| Drug Label | Travoprost is a synthetic prostaglandin F analogue. Its chemical name is [1R-[1(Z),2(1E,3R*),3,5]]-7-[3,5-Dihydroxy-2-[3-hydroxy-4-[3-(trifluoromethyl) phenoxy]-1-butenyl]cyclopentyl]-5-heptenoic acid, 1-methylethylester. It has a molecular f... |
| Active Ingredient | Travoprost |
| Dosage Form | Solution/drops; Solution |
| Route | Ophthalmic; ophthalmic |
| Strength | 0.004% |
| Market Status | Tentative Approval; Prescription |
| Company | Par Pharm |
| 3 of 4 | |
|---|---|
| Drug Name | Travatan z |
| PubMed Health | Travoprost (Into the eye) |
| Drug Classes | Antiglaucoma |
| Drug Label | Travoprost is a synthetic prostaglandin F analogue. Its chemical name is [1R-[1(Z),2(1E,3R*),3,5]]-7-[3,5-Dihydroxy-2-[3-hydroxy-4-[3-(trifluoromethyl) phenoxy]-1-butenyl]cyclopentyl]-5-heptenoic acid, 1-methylethylester. It has a molecular f... |
| Active Ingredient | Travoprost |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.004% |
| Market Status | Prescription |
| Company | Alcon Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Travoprost |
| PubMed Health | Travoprost (Into the eye) |
| Drug Classes | Antiglaucoma |
| Drug Label | Travoprost is a synthetic prostaglandin F analogue. Its chemical name is [1R-[1(Z),2(1E,3R*),3,5]]-7-[3,5-Dihydroxy-2-[3-hydroxy-4-[3-(trifluoromethyl) phenoxy]-1-butenyl]cyclopentyl]-5-heptenoic acid, 1-methylethylester. It has a molecular f... |
| Active Ingredient | Travoprost |
| Dosage Form | Solution/drops; Solution |
| Route | Ophthalmic; ophthalmic |
| Strength | 0.004% |
| Market Status | Tentative Approval; Prescription |
| Company | Par Pharm |
Travoprost ophthalmic solution is indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Travoprost is also currently indicated for the decrease of elevated intraocular pressure in paediatric patients aged 2 months to < 18 years with ocular hypertension or paediatric glaucoma.
FDA Label
Decrease of elevated intraocular pressure in adult patients with ocular hypertension or open-angle glaucoma (see section 5. 1). Decrease of elevated intraocular pressure in paediatric patients aged 3 years to < 18 years with ocular hypertension or paediatric glaucoma.
Decrease of elevated intraocular pressure in adult patients with ocular hypertension or open-angle glaucoma (see section 5. 1).
Decrease of elevated intraocular pressure in paediatric patients aged 2 months to < 18 years with ocular hypertension or paediatric glaucoma (see section 5. 1).
Treatment of glaucoma
Travoprost, an isopropyl ester prodrug, is a synthetic prostaglandin F2 alpha analog that is rapidly hydrolyzed by esterases in the cornea to its biologically active free acid. The travoprost free acid is potent and highly selective for the FP prostanoid receptor.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
S01EE04
S01EE04
S - Sensory organs
S01 - Ophthalmologicals
S01E - Antiglaucoma preparations and miotics
S01EE - Prostaglandin analogues
S01EE04 - Travoprost
Absorption
Travoprost is systemically absorbed through the cornea. In humans, peak plasma concentrations of travoprost free acid were low (25 pg/mL or less) and occurred within 30 minutes following topical ocular administration of one drop of 0.004% travoprost ophthalmic solution.
Route of Elimination
Less than 2% of the topical ocular dose of travoprost was excreted in the urine within 4 hours as the travoprost free acid. Moreover, elimination from plasma is rapid, resulting in concentrations below the limit of quantitation (< 10 pg/mL) by one hour. Furthermore, in rats, 95% of a subcutaneous radiolabeled dose was eliminated within 24 hours. The major route of elimination was via the bile (61%) with the remainder excreted by the kidneys.
Volume of Distribution
Given the data currently available, it has been recorded that travoprost free acid is moderately distributed into body tissues with a volume of distribution of 2.6 L/kg in rats.
Clearance
Data regarding the clearance of travoprost is not readily available or accessible.
Travoprost, an isopropyl ester prodrug, is hydrolyzed by esterases in the cornea to its biologically active free acid. Systemically, travoprost free acid is rapidly and extensively metabolized in the kidney, liver, and lung to inactive metabolites via beta-oxidation of the α(carboxylic acid) chain to give the 1,2-dinor and 1,2,3,4-tetranor analogs, via oxidation of the 15-hydroxyl moiety, as well as via reduction of the 13,14 double bond.
The terminal elimination half-life of travoprost free acid is determined to be approximately 45 minutes, although studies demonstrated half-life values that ranged from 17 to 86 minutes.
Travoprost, a prostaglandin F2 analogue, is a highly selective full agonist which has a high affinity for the prostaglandin FP receptor, and facilitates reductions in intraocular pressure by increasing the outflow of aqueous humour via trabecular meshwork and uveoscleral pathways. Reduction of the intraocular pressure in man starts about 2 hours after administration and maximum effect is reached after 12 hours. Significant lowering of intraocular pressure can be maintained for periods exceeding 24 hours with a single dose.
Registration Number : 227MF10153
Registrant's Address : ul. Pra(´)ce 657,277 11 Neratovice, Czech Republic
Initial Date of Registration : 2015-06-01
Latest Date of Registration : 2022-09-14
Registration Number : 227MF10136
Registrant's Address : To(´) utca 1-5. , 1045 Budapest, Hungary
Initial Date of Registration : 2015-05-12
Latest Date of Registration : 2017-01-13
Travoprost "For manufacturing only"
Registration Number : 227MF10195
Registrant's Address : 207, Sujeong-ro, Jangan-myeon, Hwaseong-si, Gyeonggi-do, 18581, Republic of Korea
Initial Date of Registration : 2015-08-03
Latest Date of Registration : 2015-08-03

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Date of Issue : 2022-09-02
Valid Till : 2025-05-05
Written Confirmation Number : WC-0349
Address of the Firm : MIs. MSN Laboratories Private Limited, Unit-II, sv. No, 50, Kardanur (Village), ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
Registrant Name : Novartis Korea Ltd.
Registration Date : 2012-11-08
Registration Number : 20121108-208-I-101-01
Manufacturer Name : Dr. Reddy's Laboratories (EU...
Manufacturer Address : Steanard Lane, Mirfield, West Yorkshire, WF14 8HZ
Registrant Name : YS Life Science Co., Ltd.
Registration Date : 2017-06-23
Registration Number : 20170623-208-I-528-02
Manufacturer Name : YS Life Science Co., Ltd.
Manufacturer Address : 207 Sujeong-ro, Jangan-myeon, Hwaseong-si, Gyeonggi-do

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : Cayman Pharma, a leading European provider of cGMP prostaglandin APIs, was formed in 2006 through the merger of Cayman Chemical and NeraPharm, both with vast expertise in prostagla...
About the Company : Chirogate, established in 1999, is a leading supplier of niche-market molecules, specializing in prostaglandins. With a focus on quality and compliance, Chirogate has built a reput...
 Century has been an API manufacturer for over 40 years & is the partner of choice for multipurpose custom manufacturing projects.
Century has been an API manufacturer for over 40 years & is the partner of choice for multipurpose custom manufacturing projects.
About the Company : Century Pharmaceuticals, established in 1982, has 40 years of experience in manufacturing APIs. It has been supplying APIs produced in-house to several major pharma companies in In...
About the Company : EUROAPI is focused on reinventing active ingredient solutions to meet the needs of customers and patients worldwide sustainably. We are a leading player in APIs with approximately ...
About the Company : Transo-Pharm, a fully licensed and certified distributor, specializes in pharmaceutical components for the health and veterinary industries. It offers support to clients throughout...
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for the pharmaceutical and biotech industries. LGM is also a full service CDMO providing formulation, ...
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
About the Company : Founded in 1984, DRL is well-known for its generic APIs and its track record in drug product development. It is one of the earliest pharma API manufacturers with a diverse portfoli...
 Gentec Pharmaceutical Group is focused on manufacturing & developing APIs/HPAPIs, Advanced Intermediates & Fine Chemicals.
Gentec Pharmaceutical Group is focused on manufacturing & developing APIs/HPAPIs, Advanced Intermediates & Fine Chemicals.
About the Company : With more than 30 years of experience, Gentec Pharmaceutical Group has established itself as one of the leaders in raw materials and ingredients for the food, dietary and nutrition...
 Capital Farma, a leading European pharmaceutical company focusing on the development & distribution of niche APIs & Pharma Services.
Capital Farma, a leading European pharmaceutical company focusing on the development & distribution of niche APIs & Pharma Services.
About the Company : Capital Farma offers a comprehensive range of pharmaceutical solutions. Our API offering includes active ingredients sourced exclusively from top European manufacturers, ensuring t...
About the Company : More than 35 years of dedication to quality, service and pursuit of excellence, CHEMO was founded by Hugo Sigman, M.D., and Silvia Gold, Biochemist, in Spain (Barcelona), in 1977, ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Company :
Travoprost
Drug Cost (USD) : 14,924,685
Year : 2022
Prescribers : 10722
Prescriptions : 37509

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Travoprost
Drug Cost (USD) : 129,313,971
Year : 2022
Prescribers : 137795
Prescriptions : 560717

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Travoprost
Drug Cost (USD) : 25,577,164
Year : 2021
Prescribers : 31978
Prescriptions : 77704

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Travoprost
Drug Cost (USD) : 144,152,907
Year : 2021
Prescribers : 157869
Prescriptions : 615678

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Travoprost
Drug Cost (USD) : 122,232,660
Year : 2020
Prescribers : 112407
Prescriptions : 387800

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Travoprost
Drug Cost (USD) : 154,098,040
Year : 2020
Prescribers : 184367
Prescriptions : 660013

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Travoprost
Drug Cost (USD) : 391,469,827
Year : 2019
Prescribers : 291128
Prescriptions : 1275031

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Travoprost
Drug Cost (USD) : 864,560
Year : 2019
Prescribers : 3433
Prescriptions : 3434

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Travoprost
Drug Cost (USD) : 393,794,154
Year : 2018
Prescribers : 317008
Prescriptions : 1426934

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Travoprost
Drug Cost (USD) : 0
Year : 2018
Prescribers :
Prescriptions : 0

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
19 Dec 2024
Reply
22 Jun 2023
Reply
04 Feb 2023
Reply
10 Jan 2022
Reply
10 Nov 2021
Reply
15 Jun 2021
Reply
01 Oct 2020
Reply
26 Dec 2019
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
63
PharmaCompass offers a list of Travoprost API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Travoprost manufacturer or Travoprost supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Travoprost manufacturer or Travoprost supplier.
PharmaCompass also assists you with knowing the Travoprost API Price utilized in the formulation of products. Travoprost API Price is not always fixed or binding as the Travoprost Price is obtained through a variety of data sources. The Travoprost Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Travoprost manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Travoprost, including repackagers and relabelers. The FDA regulates Travoprost manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Travoprost API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Travoprost manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Travoprost supplier is an individual or a company that provides Travoprost active pharmaceutical ingredient (API) or Travoprost finished formulations upon request. The Travoprost suppliers may include Travoprost API manufacturers, exporters, distributors and traders.
click here to find a list of Travoprost suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Travoprost DMF (Drug Master File) is a document detailing the whole manufacturing process of Travoprost active pharmaceutical ingredient (API) in detail. Different forms of Travoprost DMFs exist exist since differing nations have different regulations, such as Travoprost USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Travoprost DMF submitted to regulatory agencies in the US is known as a USDMF. Travoprost USDMF includes data on Travoprost's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Travoprost USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Travoprost suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Travoprost Drug Master File in Japan (Travoprost JDMF) empowers Travoprost API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Travoprost JDMF during the approval evaluation for pharmaceutical products. At the time of Travoprost JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Travoprost suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Travoprost Drug Master File in Korea (Travoprost KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Travoprost. The MFDS reviews the Travoprost KDMF as part of the drug registration process and uses the information provided in the Travoprost KDMF to evaluate the safety and efficacy of the drug.
After submitting a Travoprost KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Travoprost API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Travoprost suppliers with KDMF on PharmaCompass.
A Travoprost written confirmation (Travoprost WC) is an official document issued by a regulatory agency to a Travoprost manufacturer, verifying that the manufacturing facility of a Travoprost active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Travoprost APIs or Travoprost finished pharmaceutical products to another nation, regulatory agencies frequently require a Travoprost WC (written confirmation) as part of the regulatory process.
click here to find a list of Travoprost suppliers with Written Confirmation (WC) on PharmaCompass.
Travoprost Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Travoprost GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Travoprost GMP manufacturer or Travoprost GMP API supplier for your needs.
A Travoprost CoA (Certificate of Analysis) is a formal document that attests to Travoprost's compliance with Travoprost specifications and serves as a tool for batch-level quality control.
Travoprost CoA mostly includes findings from lab analyses of a specific batch. For each Travoprost CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Travoprost may be tested according to a variety of international standards, such as European Pharmacopoeia (Travoprost EP), Travoprost JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Travoprost USP).
