
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
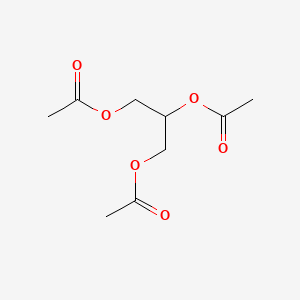

1. Enzactin
2. Triacetyl Glycerol
3. Triacetyl-glycerol
4. Triacetylglycerol
1. 102-76-1
2. Glyceryl Triacetate
3. Glycerol Triacetate
4. Enzactin
5. Glycerin Triacetate
6. Triacetine
7. Triacetylglycerol
8. Fungacetin
9. Glyped
10. Triacetyl Glycerine
11. Vanay
12. Kesscoflex Tra
13. Kodaflex Triacetin
14. 1,2,3-propanetriol, Triacetate
15. 1,2,3-triacetoxypropane
16. Acetin, Tri-
17. Propane-1,2,3-triyl Triacetate
18. 1,2,3-propanetriol, 1,2,3-triacetate
19. Triacetina
20. Triacetinum
21. 1,2,3-propanetriol Triacetate
22. Triacetin [inn]
23. Ujostabil
24. Estol 1581
25. Fema No. 2007
26. Triacetyl Glycerin
27. Triacetyl Glycerol
28. 1,2,3-propanetriyl Triacetate
29. 1,2,3-triacetylglycerol
30. 2,3-diacetyloxypropyl Acetate
31. Glyceryltriacetate
32. Nsc 4796
33. Triacetin (usp/inn)
34. Acetic, 1,2,3-propanetriyl Ester
35. Enzactin (tn)
36. Nsc-4796
37. Ins No.1518
38. 1,2,3-triacetyl-glycerol
39. 2-(acetyloxy)-1-[(acetyloxy)methyl]ethyl Acetate
40. Ins-1518
41. 1,2,3-triacetyl-sn-glycerol
42. Chebi:9661
43. Xhx3c3x673
44. E1518
45. E-1518
46. Ncgc00091612-04
47. Triacetin (1,2,3-propanetriol Triacetate)
48. Dsstox_cid_6691
49. Dsstox_rid_78184
50. Dsstox_gsid_26691
51. Fema Number 2007
52. Triacetine [inn-french]
53. Triacetinum [inn-latin]
54. Triacetina [inn-spanish]
55. Cas-102-76-1
56. Hsdb 585
57. Einecs 203-051-9
58. Triacetin (glycerol Triacetate)
59. Brn 1792353
60. Triacetin [usp:inn:ban]
61. Unii-xhx3c3x673
62. Enzacetin
63. Euzactin
64. Fungacet
65. Motisil
66. Blekin
67. Tri-acetin
68. Ai3-00661
69. Ccris 9355
70. Triacetin, Cp
71. Triacetin, Fcc
72. Triacetin, Usp
73. 3-triacetoxypropane
74. Glycerine Triacetate
75. Mfcd00008716
76. Triacetin, 99%
77. Spectrum_000881
78. Triacetin [fcc]
79. Triacetin [ii]
80. Triacetin [mi]
81. Triacetin [fhfi]
82. Triacetin [hsdb]
83. Triacetin [inci]
84. Spectrum2_000939
85. Spectrum3_001368
86. Spectrum4_000362
87. Spectrum5_001376
88. Triacetin [vandf]
89. Triacetin [mart.]
90. Ec 203-051-9
91. Triacetin, >=99.5%
92. Schembl3870
93. Triacetin [usp-rs]
94. Triacetin [who-dd]
95. Bspbio_002896
96. Glycerol Triacetate Tributyrin
97. Kbiogr_000823
98. Kbioss_001361
99. 4-02-00-00253 (beilstein Handbook Reference)
100. Mls002152946
101. 1,3-propanetriol, Triacetate
102. Divk1c_000740
103. Glyceryl Triacetate, >=99%
104. Spectrum1500585
105. Triacetin, Analytical Standard
106. Spbio_000878
107. Triacetin, 99%, Fcc, Fg
108. 1,2,3-propanediol Triethanoate
109. Chembl1489254
110. Dtxsid3026691
111. Triacetin [ep Monograph]
112. Fema 2007
113. Hms502e22
114. Kbio1_000740
115. Kbio2_001361
116. Kbio2_003929
117. Kbio2_006497
118. Kbio3_002116
119. Nsc4796
120. Triacetin [usp Monograph]
121. Ninds_000740
122. Hms1921g05
123. Hms2092o09
124. Hms2232i22
125. Pharmakon1600-01500585
126. Triacetin, >=99%, Natural, Fg
127. Hy-b0896
128. Zinc1530705
129. Tox21_111155
130. Tox21_201745
131. Tox21_300111
132. Wln: 1vo1yov1 & 1ov1
133. Ccg-39680
134. Lmgl03012615
135. Nsc757364
136. S4581
137. Triacetin, 8ci, Ban, Inn, Usan
138. 1,2,3-propanetriol Triacetate, 9ci
139. Akos009028851
140. Tox21_111155_1
141. Glyceryl Triacetate, >=99.0% (gc)
142. Nsc-757364
143. 1,3-bis(acetyloxy)propan-2-yl Acetate
144. Idi1_000740
145. Ncgc00091612-01
146. Ncgc00091612-02
147. Ncgc00091612-03
148. Ncgc00091612-05
149. Ncgc00091612-06
150. Ncgc00091612-07
151. Ncgc00091612-09
152. Ncgc00254207-01
153. Ncgc00259294-01
154. Ls-13668
155. Smr001224538
156. Sbi-0051540.p002
157. Ft-0626753
158. G0086
159. En300-19216
160. D00384
161. E 1518
162. E75962
163. Q83253
164. Ab00052112_06
165. A800614
166. Sr-05000002079
167. J-000781
168. Sr-05000002079-1
169. 2-(acetyloxy)-1-[(acetyloxy)methyl]ethyl Acetate #
170. Z1258578263
171. Triacetin, Gta F.g (1,2,3-propanetriol Triacetate)
172. Triacetin, United States Pharmacopeia (usp) Reference Standard
173. Triacetin, Pharmaceutical Secondary Standard; Certified Reference Material
174. 1,2,3-propanetriol Triacetate; Glycerol Triacetate, Usp Grade(1.03000); Triacetine; Glycerol Triacetate; Glyceryl Triacetate; Propane-1,2,3-triyl Triacetate
| Molecular Weight | 218.20 g/mol |
|---|---|
| Molecular Formula | C9H14O6 |
| XLogP3 | 0.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Exact Mass | 218.07903816 g/mol |
| Monoisotopic Mass | 218.07903816 g/mol |
| Topological Polar Surface Area | 78.9 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 229 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ Canavan disease (CD) is a fatal dysmyelinating genetic disorder associated with aspartoacylase deficiency, resulting in decreased brain acetate levels and reduced myelin lipid synthesis in the developing brain. Here we tested tolerability of a potent acetate precursor, glyceryl triacetate (GTA), at low doses in two infants diagnosed with CD, aged 8 and 13 months. Much higher doses of GTA were evaluated for toxicity in the tremor rat model of CD. GTA was given orally to the infants for up to 4.5 and 6 months, starting at 25 mg/kg twice daily, doubling the dose weekly until a maximum of 250 mg/kg reached. GTA treatment caused no detectable toxicity and the patients showed no deterioration in clinical status. Lack of GTA toxicity in two CD patients in low-dose trials suggests that higher, effective dose studies in human CD patients are warranted.
PMID:19685155 Madhavarao CN et al; J Inherit Metab Dis 32 (5): 640-50 (2009)
Antifungal Agents; Solvents
National Library of Medicine's Medical Subject Headings. Triacetin. Online file (MeSH, 2015). Available from, as of April 28, 2015: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
/EXPL THER/ The FDA approved food additive Triacetin (glyceryl triacetate, GTA) has been safely used for acetate supplementation therapy in Canavan disease, a leukodystrophy due to aspartoacylase (ASPA) mutation. This study characterized the effects of GTA on the proliferation and differentiation of six primary glioblastoma (GBM)-derived glioma stem-like cells (GSCs) relative to established U87 and U251 GBM cell lines, normal human cerebral cortical astrocytes, and murine neural stem cells. GTA reduced proliferation of GSCs greater than established GBM lines. Moreover, GTA reduced growth of the more aggressive mesenchymal GSCs greater than proneural GSCs. Although sodium acetate induced a dose-dependent reduction of GSC growth, it also reduced cell viability. GTA-mediated growth inhibition was not associated with differentiation, but increased protein acetylation. These data suggest that GTA-mediated acetate supplementation is a novel therapeutic strategy to inhibit GSC growth.
PMID:25573156 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4414874 Long PM et al; J Cell Physiol 230 (8): 1929-43 (2015)
/EXPL THER/ Canavan disease (CD) is a rare autosomal recessive neurodegenerative disorder presenting in early infancy. The course of the disease is variable, but it is always fatal. CD is caused by mutations in the ASPA gene, which codes for the enzyme aspartoacylase (ASPA), which breaks down N-acetylaspartate (NAA) to acetate and aspartic acid. The lack of NAA-degrading enzyme activity leads to excess accumulation of NAA in the brain and deficiency of acetate, which is necessary for myelin lipid synthesis. Glyceryltriacetate (GTA) is a short-chain triglyceride with three acetate moieties on a glycerol backbone and has proven an effective acetate precursor. Intragastric administration of GTA to tremor mice results in greatly increased brain acetate levels, and improved motor functions. GTA given to infants with CD at a low dose (up to 0.25 g/kg/d) resulted in no improvement in their clinical status, but also no detectable toxicity. We present for the first time the safety profile of high dose GTA (4.5 g/kg/d) in 2 patients with CD. We treated 2 infants with CD at ages 8 months and 1 year with high dose GTA, for 4.5 and 6 months respectively. No significant side effects and no toxicity were observed. Although the treatment resulted in no motor improvement, it was well tolerated. The lack of clinical improvement might be explained mainly by the late onset of treatment, when significant brain damage was already present. Further larger studies of CD patients below age 3 months are required in order to test the long-term efficacy of this drug.
PMID:21474353 Segel R et al; Mol Genet Metab 103 (3): 203-6 (2011)
For more Therapeutic Uses (Complete) data for TRIACETIN (9 total), please visit the HSDB record page.
For external use only: Not for ophthalmic use.
Novak, K.M. (ed.). Drug Facts and Comparisons 59th Edition 2005. Wolters Kluwer Health. St. Louis, Missouri 2005., p. 2024
Irritation or sensitivity: Discontinue treatment and notify physician.
Novak, K.M. (ed.). Drug Facts and Comparisons 59th Edition 2005. Wolters Kluwer Health. St. Louis, Missouri 2005., p. 2024
Diabetics or patients with impaired blood circulation: Use spray with caution.
Novak, K.M. (ed.). Drug Facts and Comparisons 59th Edition 2005. Wolters Kluwer Health. St. Louis, Missouri 2005., p. 2024
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
Solvents
Liquids that dissolve other substances (solutes), generally solids, without any change in chemical composition, as, water containing sugar. (Grant and Hackh's Chemical Dictionary, 5th ed) (See all compounds classified as Solvents.)
...Triacetin is more rapidly absorbed from the gastrointestinal tract in 3 hours than the other fats tested. Triacetin has been shown to be a source of liver glycogen and when fed in amounts equal in caloric value to 15% glucose it was utilized as efficiently as was glucose.
WHO; Food Additive Series 8: Toxicological evaluation of some food colours, thickening agents, and certain other substancse (1975). Available from, as of February 15, 2005: https://www.inchem.org/documents/jecfa/jecmono/v08je12.htm
Mongrel dogs /were used/ to determine the systemic, hindlimb, gut, hepatic, and renal uptake of acetate during infusion of a 5% v/v aqueous solution of triacetin. A primed, continuous infusion of [1-(14)C]-acetate was continued for 7 hr with 10 animals. Three hours after the start of the tracer infusion, the animals were infused with triacetin at a rate of 47 umol/kg/min for 4 hr. Blood and breath samples were taken at 15-min intervals for the last 30 min. Steady-state conditions were achieved in plasma acetate concentrations and specific activity and in expired [(14)-C02]. Plasma acetate concentrations were 1180, 935, 817, 752, and 473 umol/L /(all values approximate)/ in the aorta, renal vein, portal vein, femoral vein, and hepatic vein, respectively. The acetate turnover rate during triacetin infusion was 2214 umol/min; systemic acetate turnover accounted for 68% of triacetin-derived acetate.
Cosmetic Ingredient Review Expert Panel; International Journal of Toxicology: 22(suppl. 2): 1-10 (2003)
Triacetin has been administered iv to mongrel dogs. The majority of infused triacetin underwent intravascular hydrolysis, and the majority of the resulting acetate is oxidized. Triacetin was found to be hydrolyzed by human intestinal lipase.
Sheftel, V.O.; Indirect Food Additives and Polymers. Migration and Toxicology. Lewis Publishers, Boca Raton, FL. 2000., p. 284
...Triacetin is rapidly hydrolysed in vitro by all tissues of the organism including the gastrointestinal tract.
WHO; Food Additive Series 8: Toxicological evaluation of some food colours, thickening agents, and certain other substancse (1975). Available from, as of February 15, 2005: https://www.inchem.org/documents/jecfa/jecmono/v08je12.htm
Groups of female mongrel dogs to study the metabolic effects of isocaloric and hypercaloric infusions of 5% v/v aqueous triacetin. A primed, continuous infusion of 5 umol/kg (0.3 uCi/kg/min) [(13)C]-acetoacetate and 1.0 uCi/kg (0.01 uCi/kg/min) [(3)H]-glucose was continued for 6 hr. Three hours after the start of the isotope infusion, dosing with triacetin was started. Six animals were infused at a rate of 47 umol/kg/min and seven were infused at a rate of 70 umol/kg/min triacetin for 3 hr. Blood and breath samples were taken at 15 to 30-min intervals. A group of four animals was infused with 70 umol/kg/min glycerol and used as the control for the hypercaloric infusion. During isocaloric infusion of triacetin, plasma acetate and free fatty acid concentrations were significantly increased at 30 and 60 min, respectively, and remained elevated. During hypercaloric infusion, plasma acetate concentration increased progressively throughout the study, whereas the plasma free fatty acid concentration did not change. Plasma pyruvate and lactate concentrations were significantly decreased after 30 and 90 min, respectively, and throughout the study with both isocaloric and hypercaloric infusion. The plasma insulin concentrations were modestly increased during both infusions. Plasma glucose concentration was significantly decreased during isocaloric triacetin infusion; a slight but significant increase was observed with hypercaloric infusion. Glucose clearance decreased significantly in both groups during the last hour of triacetin infusion. Plasma ketone body concentrations increased significantly by 60 min, and they remained elevated with isocaloric infusion and increased progressively with hypercaloric infusion of triacetin; the increased concentrations were due to increased ketone body production. During the last hour of infusion, resting energy expenditure was significantly increased with isocaloric triacetin.
Cosmetic Ingredient Review Expert Panel; International Journal of Toxicology: 22(suppl. 2): 1-10 (2003)
Esterases in fungi or in serum act at pH >4 to slowly release acetic acid in situ. Extent of hydrolysis is automatically limited by increased acidity and lowering of pH.
Patty, F. (ed.). Industrial Hygiene and Toxicology: Volume II: Toxicology. 2nd ed. New York: Interscience Publishers, 1963., p. 1871
... The fungistatic activity of triacetin (glyceryl triacetate) results from its hydrolysis by fungalesterases to acetic acid.
Ullmann's Encyclopedia of Industrial Chemistry. 6th ed.Vol 1: Federal Republic of Germany: Wiley-VCH Verlag GmbH & Co. 2003 to Present, p. V10 391 (2003)
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 11292
Submission : 1995-01-05
Status : Inactive
Type : IV



 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
83
PharmaCompass offers a list of Triacetin API API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Triacetin API manufacturer or Triacetin API supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Triacetin API manufacturer or Triacetin API supplier.
PharmaCompass also assists you with knowing the Triacetin API API Price utilized in the formulation of products. Triacetin API API Price is not always fixed or binding as the Triacetin API Price is obtained through a variety of data sources. The Triacetin API Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Triacetin API manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Triacetin API, including repackagers and relabelers. The FDA regulates Triacetin API manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Triacetin API API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Triacetin API manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Triacetin API supplier is an individual or a company that provides Triacetin API active pharmaceutical ingredient (API) or Triacetin API finished formulations upon request. The Triacetin API suppliers may include Triacetin API API manufacturers, exporters, distributors and traders.
click here to find a list of Triacetin API suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Triacetin API DMF (Drug Master File) is a document detailing the whole manufacturing process of Triacetin API active pharmaceutical ingredient (API) in detail. Different forms of Triacetin API DMFs exist exist since differing nations have different regulations, such as Triacetin API USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Triacetin API DMF submitted to regulatory agencies in the US is known as a USDMF. Triacetin API USDMF includes data on Triacetin API's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Triacetin API USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Triacetin API suppliers with USDMF on PharmaCompass.
Triacetin API Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Triacetin API GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Triacetin API GMP manufacturer or Triacetin API GMP API supplier for your needs.
A Triacetin API CoA (Certificate of Analysis) is a formal document that attests to Triacetin API's compliance with Triacetin API specifications and serves as a tool for batch-level quality control.
Triacetin API CoA mostly includes findings from lab analyses of a specific batch. For each Triacetin API CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Triacetin API may be tested according to a variety of international standards, such as European Pharmacopoeia (Triacetin API EP), Triacetin API JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Triacetin API USP).
