
Synopsis
Synopsis
0
CEP/COS
0
VMF
0
Australia

0
Listed Dossiers
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Apo Triazo
2. Apo-triazo
3. Gen Triazolam
4. Gen-triazolam
5. Halcion
6. Trilam
7. U 33,030
8. U-33,030
9. U33,030
1. Halcion
2. 28911-01-5
3. Songar
4. Triazolamum [inn-latin]
5. U-33,030
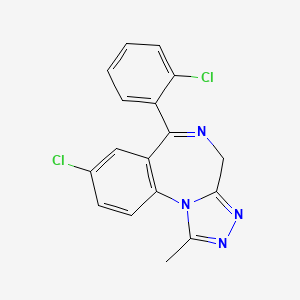
6. 8-chloro-6-(2-chlorophenyl)-1-methyl-4h-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
7. Dea No. 2887
8. Triazolam Civ
9. U 33030
10. U-33030
11. N05cd05
12. 8-chloro-6-(o-chlorophenyl)-1-methyl-4h-s-triazolo(4,3-a)(1,4)benzodiazepine
13. Clorazolam
14. Novidorm
15. Chembl646
16. 4h-(1,2,4)triazolo(4,3-a)(1,4)benzodiazepine, 8-chloro-6-(2-chlorophenyl)-1-methyl-
17. Chebi:9674
18. 8-chloro-6-(2-chlorophenyl)-1-methyl-4h-(1,2,4)triazolo(4,3-a)(1,4)benzodiazepine
19. 1hm943223r
20. Ncgc00168258-01
21. Triazolamum
22. Novodorm
23. Trilam
24. 8-chloro-6-(o-chlorophenyl)-1-methyl-4h-s-triazolo[4,3-a][1,4]benzodiazepine
25. 4h-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine, 8-chloro-6-(2-chlorophenyl)-1-methyl-
26. 4h-s-triazolo(4,3-a)(1,4)benzodiazepine, 8-chloro-6-(o-chlorophenyl)-1-methyl-
27. Hypnostat
28. Halcion (tn)
29. Halcion (triazolam)
30. Ccris 1932
31. Hsdb 6759
32. Einecs 249-307-3
33. Tgar01h
34. Brn 1226643
35. Triazolam (jan/usp/inn)
36. Hypam
37. Unii-1hm943223r
38. Triazolam [usan:usp:inn:ban:jan]
39. Triazolam [inn]
40. Triazolam [jan]
41. Triazolam [mi]
42. Triazolam [hsdb]
43. Triazolam [usan]
44. Triazolam [vandf]
45. Triazolam [mart.]
46. Triazolam [who-dd]
47. Dsstox_cid_26763
48. Dsstox_rid_81886
49. Dsstox_gsid_46763
50. Schembl29228
51. Mls003899243
52. Gtpl7313
53. Zinc2212
54. Triazolam [orange Book]
55. Triazolam Civ [usp-rs]
56. Dtxsid6046763
57. Triazolam [usp Monograph]
58. Triazolam 0.1 Mg/ml In Methanol
59. Triazolam 1.0 Mg/ml In Methanol
60. Hy-b0694
61. Tox21_112617
62. 28911-01-5 (free)
63. Bdbm50001765
64. Db00897
65. Smr000058837
66. Cas-28911-01-5
67. D00387
68. U33030
69. 911t015
70. L000796
71. Q412143
72. Sr-01000937604
73. Sr-01000937604-2
74. Triazolam, European Pharmacopoeia (ep) Reference Standard
75. 8-chloro-1-methyl-6-(o-chlorophenyl)-4h-s-triazolo-[4,3-a][1,4] Benzodiazepine
76. 8-chloro-1-methyl-6-(o-chlorophenyl)-4h-s-triazolo[4,3-a][1,4]benzodiazepine
77. 8-chloro-6-(2-chlorophenyl)-1-methyl-4h-benzo[f][1,2,4]triazolo[4,3-a][1,4]diazepine
78. 8-chloro-6-(2-chlorophenyl)-1-methyl-4h-s-triazolo[4,3-a][1,4]-benzodiazepine
79. Triazolam Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
80. (e)-8-chloro-6-(2-chlorophenyl)-1-methyl-4h-benzo[f][1,2,4]triazolo[4,3-a][1,4]diazepine
81. 12-chloro-9-(2-chlorophenyl)-3-methyl-2,4,5,8-tetraazatricyclo[8.4.0.0^{2,6}]tetradeca-1(10),3,5,8,11,13-hexaene
| Molecular Weight | 343.2 g/mol |
|---|---|
| Molecular Formula | C17H12Cl2N4 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 342.0439018 g/mol |
| Monoisotopic Mass | 342.0439018 g/mol |
| Topological Polar Surface Area | 43.1 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 472 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Halcion |
| PubMed Health | Triazolam (By mouth) |
| Drug Classes | Hypnotic |
| Drug Label | HALCION Tablets contain triazolam, a triazolobenzodiazepine hypnotic agent.Triazolam is a white crystalline powder, soluble in alcohol and poorly soluble in water. It has a molecular weight of 343.21.The chemical name for triazolam is 8-chloro-6-(o-c... |
| Active Ingredient | Triazolam |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.25mg; 0.125mg |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 2 of 4 | |
|---|---|
| Drug Name | Triazolam |
| PubMed Health | Triazolam (By mouth) |
| Drug Classes | Hypnotic |
| Drug Label | Triazolam tablets contain triazolam, a triazolobenzodiazepine hypnotic agent.Triazolam is a white crystalline powder, soluble in alcohol and poorly soluble in water. It has a molecular weight of 343.21.The chemical name for triazolam is 8-chloro-6-(o... |
| Active Ingredient | Triazolam |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.25mg; 0.125mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Roxane |
| 3 of 4 | |
|---|---|
| Drug Name | Halcion |
| PubMed Health | Triazolam (By mouth) |
| Drug Classes | Hypnotic |
| Drug Label | HALCION Tablets contain triazolam, a triazolobenzodiazepine hypnotic agent.Triazolam is a white crystalline powder, soluble in alcohol and poorly soluble in water. It has a molecular weight of 343.21.The chemical name for triazolam is 8-chloro-6-(o-c... |
| Active Ingredient | Triazolam |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.25mg; 0.125mg |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 4 of 4 | |
|---|---|
| Drug Name | Triazolam |
| PubMed Health | Triazolam (By mouth) |
| Drug Classes | Hypnotic |
| Drug Label | Triazolam tablets contain triazolam, a triazolobenzodiazepine hypnotic agent.Triazolam is a white crystalline powder, soluble in alcohol and poorly soluble in water. It has a molecular weight of 343.21.The chemical name for triazolam is 8-chloro-6-(o... |
| Active Ingredient | Triazolam |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.25mg; 0.125mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Roxane |
Adjuvants, Anesthesia; GABA Modulators; Sedatives, Nonbarbiturate
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
... Triazolam /is/ indicated for the short-term treatment of insomnia characterized by difficulty in falling asleep, frequent nocturnal awakenings, and/or early morning awakenings. ... Failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric or medical illness. Worsening of insomnia or the emergence of new abnormalities of thinking or behavior may be the consequence of an unrecognized psychiatric or physical disorder. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 519
Benzodiazepine agents are widely prescribed in the treatment of anxiety and nervousness. /Benzodiazepine agents/
Amdur, M.O., J. Doull, C.D. Klaasen (eds). Casarett and Doull's Toxicology. 4th ed. New York, NY: Pergamon Press, 1991., p. 932
Pregnancy risk category: X /CONTRAINDICATED IN PREGNANCY. Studies in animals or humans, or investigational or post-marketing reports, have demonstrated positive evidence of fetal abnormalities or risk which clearly outweights any possible benefit to the patient./
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 251
In patients who have received prolonged triazolam therapy, abrupt discontinuance of the drug should be avoided since manifestations of withdrawal can be precipitated. While the risk and severity of withdrawal manifestations are increased as the duration and dose increase, such manifestations are increased as the duration and dose increase, such manifestations can occur at usual doses given for relatively short periods (e.g., 1-2 weeks) and occasionally between nightly doses. Therefore, if the drug is to be discontinued in any patient receiving more than the lowest dose for longer than a few weeks, it si recommended that dosage be tapered gradually. Gradual tapering is particularly important in patients with a seizure history. There also is evidence that abrupt discontinuance of triazolam after relatively short periods of therapy (e.g., 1 week) can result in rebound insomnia, which generally persists for one to two nights. Therefore, some clinicians suggest that gradual dosage reduction (e.g., over several nights) also be considered when discontinuing short-term triazolam therapy, since the development of rebound insomnia can perpetuate continued use of hypnotics in patients with insomnia.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2402
Urticaria, rash, pruritus, photosensitivity, immediate hypersensitivity reactions, hypotension, nonthrombocytopenic purpura, and edema may occur in patients receiving benzodiazepines. Paresthesia and lupus-like syndrome have been reported. /Benzodiazepines/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2376
Increased or decreased libido, menstrual irregularities, failure to ovulate, gynecomastia, and galactorrhea have been reported in patients receiving benzodiazepines. Genitourinary complaints such as urinary retention, difficulty in micturition, and urinary incontinence have occurred. ... /Benzodiazepines/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2376
For more Drug Warnings (Complete) data for TRIAZOLAM (15 total), please visit the HSDB record page.
For the short-term treatment of insomnia.
A short-acting benzodiazepine used as a hypnotic agent in the treatment of insomnia. Some countries temporarily withdrew triazolam from the market because of concerns about adverse reactions, mostly psychological, associated with higher dose ranges. Its use at lower doses with appropriate care and labeling has been reaffirmed by the FDA and most other countries. Triazolam has a shorter half-life than chlordiazepoxide, flurazepam, and prazepam and does not generate active metabolites.
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)
N05CD05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CD - Benzodiazepine derivatives
N05CD05 - Triazolam
Absorption
Bioavailability is 44% (oral) and 53% (sublingual).
Route of Elimination
Triazolam and its metabolites, principally as conjugated glucuronides, which are presumably inactive, are excreted primarily in the urine. Only small amounts of unmetabolized triazolam appear in the urine. The two primary metabolites accounted for 79.9% of urinary excretion.
Plasma half-life, elimination coefficient, concentration, and apparent volume of distribution were calculated at steady state and mean values were 53 hr, 0.0147/hr, 884 ng/mL, & 1.13 l/kg /respectively/.
POST C ET AL; PSYCHOPHARMACOLOGY (BERLIN) 53 (2): 105-9 (1977)
Triazolam is rapidly and nearly completely absorbed from the GI tract. It has a biphasic half-life with a reported mean apparent half-life of 3.4 hr for the initial phase and 7.8 hr for the terminal phase. It is reported to be extensively bound to plasma proteins. It is excreted in the urine in the form of its metabolites with only small amounts appearing unchanged.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 818
In a study of triazolam tablets and a liquid formulation in healthy subjects, the bioavailability of the tablets was rapid and, relative to the liquid formulation, complete. The average half-life for absorption was 8 minutes with peak concentrations being achieved an average of 42 minutes after dosing.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 818
It is not known whether triazolam is distributed into milk in humans; however, the drug and its metabolites are distributed into milk in rats.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2402
Hepatic. Small amounts of unmetabolized triazolam appear in the urine.
Triazolam undergoes hepatic microsomal oxidation to inactive hydroxylated metabolites that are eliminated primarily as glucuronide conjugates.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 520
Triazolam has known human metabolites that include 4-Hydroxytriazolam and alpha-Hydroxytriazolam.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
1.5-5.5 hours
It has a biphasic half-life with a reported mean apparent half-life of 3.4 hr for the initial phase and 7.8 hr for the terminal phase.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 818
Elimination half-life for triazolam is 1.6-5.4 hr. /From table/
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 818
Benzodiazepines bind nonspecifically to bezodiazepine receptors BNZ1, which mediates sleep, and BNZ2, which affects affects muscle relaxation, anticonvulsant activity, motor coordination, and memory. As benzodiazepine receptors are thought to be coupled to gamma-aminobutyric acid-A (GABAA) receptors, this enhances the effects of GABA by increasing GABA affinity for the GABA receptor. Binding of GABA to the site opens the chloride channel, resulting in a hyperpolarized cell membrane that prevents further excitation of the cell.
In animals, benzodiazepines protect against seizures induced by electrical stimulation and by pentylenetetrazol; benzodiazepines appear to act, at least partly, by augmenting presynaptic inhibition. The drugs suppress the spread of seizure activity but do not abolish the abnormal discharge from a focus in experimental models of epilepsy. In usual doses, benzodiazepines appear to have very little effect on the autonomic nervous system, respiration, or the cardiovascular system. /Benzodiazepines/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2379
... /Benzodiazepines/ appear to act at the limbic, thalamic, and hypothalamic levels of the CNS, producing anxiolytic, sedative, hypnotic, skeletal muscle relaxant, and anticonvulsant effects. The effects of benzodiazepines may be mediated through the inhibitory neurotransmitter gamma-aminobutyric acid. Benzodiazepines are capable of producing all levels of CNS depression from mild sedation to hypnosis to coma. /Benzodiazepines/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2379
Anxiolytic and possibly paradoxical CNS stimulatory effects of benzodiazepines are postulated to result from release of previously suppressed responses (disinhibition). After usual doses of benzodiazepines for several days, the drugs cause a moderate decrease in rapid eye movement (REM) sleep. REM rebound does not occur when the drugs are withdrawn. Stage 3 and 4 sleep are markedly reduced by usual doses of the drugs; the clinical importance of these sleep stage alterations has not been established. /Benzodiazepines/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2379
Benzodiazepines appear to produce skeletal muscle relaxation predominantly by inhibiting spinal polysynaptic afferent pathways, but the drugs may also inhibit monosynaptic afferent pathways. The drugs may inhibit monosynaptic and polysynaptic reflexes by acting as inhibitory neuronal transmitters or by blocking exitatory synaptic transmission. The drugs may also directly depress motor nerve and muscle function. /Benzodiazepines/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2379

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
56
PharmaCompass offers a list of Triazolam API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Triazolam manufacturer or Triazolam supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Triazolam manufacturer or Triazolam supplier.
PharmaCompass also assists you with knowing the Triazolam API Price utilized in the formulation of products. Triazolam API Price is not always fixed or binding as the Triazolam Price is obtained through a variety of data sources. The Triazolam Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Triazolam manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Triazolam, including repackagers and relabelers. The FDA regulates Triazolam manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Triazolam API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Triazolam manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Triazolam supplier is an individual or a company that provides Triazolam active pharmaceutical ingredient (API) or Triazolam finished formulations upon request. The Triazolam suppliers may include Triazolam API manufacturers, exporters, distributors and traders.
click here to find a list of Triazolam suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Triazolam DMF (Drug Master File) is a document detailing the whole manufacturing process of Triazolam active pharmaceutical ingredient (API) in detail. Different forms of Triazolam DMFs exist exist since differing nations have different regulations, such as Triazolam USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Triazolam DMF submitted to regulatory agencies in the US is known as a USDMF. Triazolam USDMF includes data on Triazolam's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Triazolam USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Triazolam suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Triazolam Drug Master File in Japan (Triazolam JDMF) empowers Triazolam API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Triazolam JDMF during the approval evaluation for pharmaceutical products. At the time of Triazolam JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Triazolam suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Triazolam Drug Master File in Korea (Triazolam KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Triazolam. The MFDS reviews the Triazolam KDMF as part of the drug registration process and uses the information provided in the Triazolam KDMF to evaluate the safety and efficacy of the drug.
After submitting a Triazolam KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Triazolam API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Triazolam suppliers with KDMF on PharmaCompass.
A Triazolam written confirmation (Triazolam WC) is an official document issued by a regulatory agency to a Triazolam manufacturer, verifying that the manufacturing facility of a Triazolam active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Triazolam APIs or Triazolam finished pharmaceutical products to another nation, regulatory agencies frequently require a Triazolam WC (written confirmation) as part of the regulatory process.
click here to find a list of Triazolam suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Triazolam as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Triazolam API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Triazolam as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Triazolam and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Triazolam NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Triazolam suppliers with NDC on PharmaCompass.
Triazolam Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Triazolam GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Triazolam GMP manufacturer or Triazolam GMP API supplier for your needs.
A Triazolam CoA (Certificate of Analysis) is a formal document that attests to Triazolam's compliance with Triazolam specifications and serves as a tool for batch-level quality control.
Triazolam CoA mostly includes findings from lab analyses of a specific batch. For each Triazolam CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Triazolam may be tested according to a variety of international standards, such as European Pharmacopoeia (Triazolam EP), Triazolam JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Triazolam USP).
