
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
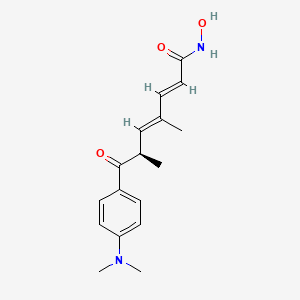

1. 2,4-heptadienamide, 7-(4-(dimethylamino)phenyl)-n-hydroxy-4,6-dimethyl-7-oxo-
2. 7-(4-(dimethylamino)phenyl)-n-hydroxy-4,6-dimethyl-7-oxo-2,4-heptadienamide
3. A 300
4. Tsa Antibioitc
1. 58880-19-6
2. Trichostatin
3. Trichostatin A (tsa)
4. Tsa
5. (2e,4e,6r)-7-[4-(dimethylamino)phenyl]-n-hydroxy-4,6-dimethyl-7-oxohepta-2,4-dienamide
6. Antibiotic A-300
7. (r)-trichostatin A
8. Chebi:46024
9. C17h22n2o3
10. (r,2e,4e)-7-(4-(dimethylamino)phenyl)-n-hydroxy-4,6-dimethyl-7-oxohepta-2,4-dienamide
11. Gnf-pf-1011
12. 3x2s926l3z
13. 58880-19-6 (r-isomer)
14. 2,4-heptadienamide, 7-(4-(dimethylamino)phenyl)-n-hydroxy-4,6-dimethyl-7-oxo-
15. 7-(4-(dimethylamino)phenyl)-n-hydroxy-4,6-dimethyl-7-oxo-2,4-heptadienamide
16. A-300-i
17. (2e,4e,6r)-7-(4-(dimethylamino)phenyl)-n-hydroxy-4,6-dimethyl-7-oxo-2,4-heptadienamide
18. [r-(e,e)]-7-[4-(dimethylamino)phenyl]-n-hydroxy-4,6-dimethyl-7-oxo-2,4-heptadienamide
19. Trichostatin-a
20. Tricostatin A
21. 7-[4-(dimethylamino)phenyl]-n-hydroxy-4,6-dimethyl-7-oxo-2,4-heptadienamide
22. 2,4-heptadienamide, 7-(4-(dimethylamino)phenyl)-n-hydroxy-4,6-dimethyl-7-oxo-, (2e,4e,6r)-
23. 2,4-heptadienamide, 7-[4-(dimethylamino)phenyl]-n-hydroxy-4,6-dimethyl-7-oxo-, (2e,4e,6r)-
24. Tsn
25. Trichostatina
26. Trichostatine A
27. Unii-3x2s926l3z
28. Trichlostatin A
29. Trichostatin(s)
30. Dtxsid6037063
31. Nsc-311042
32. (2e,4e,6r)-7-(4-dimethylaminophenyl)-4,6-dimethyl-7-oxo-hepta-2,4-dienehydroxamic Acid
33. (2e,4e,6r)-7-(4-dimethylaminophenyl)-n-hydroxy-4,6-dimethyl-7-oxo-hepta-2,4-dienamide
34. Trichostatin A,tsa
35. Mfcd03848392
36. Ncgc_tsa
37. 1c3r
38. 3f0r
39. Trichostatin-a - Tsa
40. Trichostatin A [mi]
41. Schembl19886
42. Mls006011095
43. Sgcto-002
44. Schembl675951
45. Gtpl7005
46. Chebi:93196
47. Vtr-297
48. Bcpp000035
49. Hms1362l09
50. Hms1792l09
51. Hms1990l09
52. Hms3403l09
53. Hms3649o20
54. Bcp01776
55. Ex-a1665
56. Trichostatin A, Ready Made Solution
57. Bdbm50005711
58. Lmpk01000055
59. S1045
60. Trichostatin A From Streptomyces Sp.
61. Akos015899840
62. Zinc100014731
63. Ccg-208142
64. Ccg-208681
65. Cs-0499
66. Db04297
67. Nsc 311042
68. Ncgc00162453-01
69. Ncgc00162453-02
70. Ncgc00162453-03
71. Ncgc00162453-04
72. Ncgc00162453-05
73. Ncgc00162453-15
74. 3c10
75. Ac-35470
76. As-74315
77. Hy-15144
78. Smr004702883
79. A8183
80. Sw219664-1
81. T2477
82. A25618
83. M02571
84. 880t196
85. Q425894
86. Sr-05000013796
87. Q-201864
88. Sr-05000013796-3
89. Brd-k68202742-001-04-1
90. Brd-k68202742-001-05-8
91. Trichostatin A, >=98% (hplc), From Streptomyces Sp.
92. Trichostatin A, Streptomyces Sp. - Cas 58880-19-6
93. Trichostatin A??, Vetec(tm) Reagent Grade, From Streptomyces Sp., >=98%
94. (2e,4e,6r)-7-(4-(dimethylamino)phen Yl)-n-hydroxy-4,6-dimethyl-7-oxo-2,4-heptadienamid E
95. (6r)-n-hydroxy-4,6-dimethyl-7-oxo-7-[4-(dimethylamino)phenyl]-2,4-heptadienamide
96. 7-[4-(dimethylamino)phenyl]-n-hydroxy-4,6r-dimethyl-7-oxo-2e,4e-heptadienamide
97. 2,4-heptadienamide, 7-(4-(dimethylamino)phenyl)-n-hydroxy-4,6-dimethyl-7-oxo-,(2e,4e,6r)-
98. 2,4-heptadienamide, 7-[4-(dimethylamino)phenyl]-n-hydroxy-4,6-dimethyl-7-oxo-, (2e,4e,6r)- (9ci)
99. 2,4-heptadienamide, 7-[4-(dimethylamino)phenyl]-n-hydroxy-4,6-dimethyl-7-oxo-, [r-(e,e)]-
100. 2,4-heptadienamide,7-[4-(dimethylamino)phenyl]-n-hydroxy-4,6-dimethyl-7-oxo-, (2e,4e,6r)-
| Molecular Weight | 302.37 g/mol |
|---|---|
| Molecular Formula | C17H22N2O3 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 302.16304257 g/mol |
| Monoisotopic Mass | 302.16304257 g/mol |
| Topological Polar Surface Area | 69.6 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 447 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
Histone Deacetylase Inhibitors
Compounds that inhibit HISTONE DEACETYLASES. This class of drugs may influence gene expression by increasing the level of acetylated HISTONES in specific CHROMATIN domains. (See all compounds classified as Histone Deacetylase Inhibitors.)
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?