
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
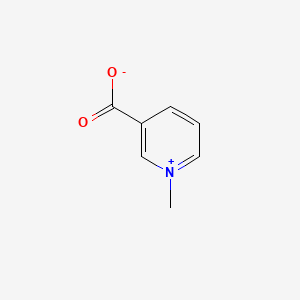

1. Trigonelline Chloride
2. Trigonelline Iodide
3. Trigonelline Ion
4. Trigonelline Tosylate
1. 535-83-1
2. Trigenolline
3. N-methylnicotinate
4. Caffearine
5. Gynesine
6. Coffearine
7. Betain Nicotinate
8. 1-methylpyridinium-3-carboxylate
9. Trigonellin
10. Coffearin
11. Betaine Nicotinate
12. 1-methylpyridinio-3-carboxylate
13. Nicotinic Acid N-methylbetaine
14. 1-methylpyridin-1-ium-3-carboxylate
15. N-methylnicotinic Acid
16. Trigenelline
17. Caffearin
18. 1-methyl-3-pyridiniumcarboxylate
19. 3-carboxy-1-methylpyridinium Hydroxide Inner Salt
20. N'-methylnicotinate
21. Chebi:18123
22. 3nq9n60i00
23. Pyridinium, 3-carboxy-1-methyl-, Hydroxide, Inner Salt
24. 1-methyl-nicotinic Acid Anion(trigonelline)
25. 3-carboxy-1-methylpyridinium Hydroxide, Inner Salt
26. 1-methylpyridin-1-ium-3-carboxylic Acid
27. Ccris 1332
28. Einecs 208-620-5
29. Brn 3905114
30. Unii-3nq9n60i00
31. Hsdb 7684
32. 1-methylnicotinate
33. Trigonelline,(s)
34. N-methyl-nicotinate
35. 3-carboxy-1-methylpyridinium Inner Salt
36. N'-methylnicotinic Acid
37. 6138-40-5
38. Trigonelline [mi]
39. Trigonelline [hsdb]
40. 5-22-02-00143 (beilstein Handbook Reference)
41. Mls002153895
42. N-methylnicotinic Acid Betaine
43. Schembl195666
44. Spectrum1500880
45. Trigonelline [usp-rs]
46. Chembl350675
47. Dtxsid2026230
48. Hms2096h08
49. Hms2234k22
50. Hms3371k21
51. Hy-n0414
52. Bdbm50548713
53. Ccg-38517
54. Mfcd00054262
55. Nsc714350
56. Akos005067859
57. Nsc-714350
58. Sdccgmls-0066739.p001
59. Ncgc00095649-01
60. Ncgc00095649-02
61. Ncgc00095649-03
62. Ncgc00095649-04
63. Ncgc00095649-07
64. Ac-34290
65. As-17722
66. Smr001233244
67. Ab00052974
68. Cs-0008944
69. Ft-0689338
70. N2788
71. 1-methyl-5-(oxylatocarbonyl)pyridinium-3-ide
72. Pyridinium, 3-carboxy-1-methyl-, Inner Salt
73. A14825
74. C01004
75. 3-carboxy-1-methyl-pyridinium Hydroxide Inner Salt
76. Q928965
77. 3-carboxy-1-methylpyridinium, Hydroxide, Inner Salt
78. W-105723
79. Trigonelline (constituent Of Fenugreek Seed) [dsc]
| Molecular Weight | 137.14 g/mol |
|---|---|
| Molecular Formula | C7H7NO2 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 137.047678466 g/mol |
| Monoisotopic Mass | 137.047678466 g/mol |
| Topological Polar Surface Area | 44 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 130 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ Fenugreek seeds are known for their characteristic smell of soup seasoning and as an ingredient of Indian curry. Traditionally the seeds are used as macerate for the treatment of diabetes, cough, and flatulence, to increase breast milk secretion, and for anti-inflammatory and aphrodisiac effects. The use is limited by its unpleasant smell and bitter taste which can be modified by adding mint leaves to the macerate. Antidiabetic properties are attributed mainly to galactomannan, 4-hydroxyisoleucin (4-OH-Ile), diosgenin and trigonelline. These substances demonstrate direct antidiabetic properties in clinical studies by increasing insulin secretion (4-OH-Ile), decreasing insulin resistance and glucose resorption from the GIT (galactomannan) and improvement in B-cells regeneration (trigonelline). Besides this main effect, the herb improves blood lipid spectre (4-OH-Ile, diosgenin), and has reno-protective (4-OH-Ile, trigonelline), neuroprotective (trigonelline) and antioxidant (diosgenin, trigonelline) effects. Antidiabetic efficacy of trigonelline is comparable to glibenclamide treatment and more effective than sitagliptine therapy. Given the large body of evidence and promising results in comparison with standard pharmacotherapy, fenugreek active substances have a potential to become a source of new antidiabetic medication.Key words: fenugreek Trigonella foenum-graecum diabetes mellitus type 2 biological activity.
PMID:26400229 Koupy D et al; Ceska Slov Farm 64 (3): 67-71 (2015)
/EXPL THER/ There is evidence that Trigonella foenum-graecum L. (fenugreek), a traditional Chinese herb, and its components are beneficial in the prevention and treatment of diabetes and central nervous system disease. The pharmacological activities of trigonelline, a major alkaloid component of fenugreek, have been more thoroughly evaluated than fenugreek's other components, especially with regard to diabetes and central nervous system disease. Trigonelline has hypoglycemic, hypolipidemic, neuroprotective, antimigraine, sedative, memory-improving, antibacterial, antiviral, and anti-tumor activities, and it has been shown to reduce diabetic auditory neuropathy and platelet aggregation. It acts by affecting beta cell regeneration, insulin secretion, activities of enzymes related to glucose metabolism, reactive oxygen species, axonal extension, and neuron excitability. However, further study of trigonelline's pharmacological activities and exact mechanism is warranted, along with application of this knowledge to its clinical usage. This review aims to give readers a survey of the pharmacological effects of trigonelline, especially in diabetes, diabetic complications and central nervous system disease. In addition, because of its pharmacological value and low toxicity, the reported adverse effects of trigonelline in experimental animal models and humans are briefly reviewed, and the pharmacokinetics of trigonelline are also discussed.
PMID:22680628 Zhou J et al; Curr Med Chem. 2012;19(21):3523-31 (2012)
... The concentration-time curves of trigonelline in rabbits after ... iv administration were shown to fit one-compartment and two-compartment open model, respectively. The main parameters after iv /administration/ of trigonelline were as follows: T1/2 alpha was 10.8 min, T1/2 beta was 44.0 min, K21 was 0.044 min-1, K10 was 0.026 min-1, K12 was 0.017 min-1, AUC was 931.0 mg.min/L . /It was concluded that/ trigonelline showed a middle rate of absorption and fast rate of elimination in rabbit...
PMID:12889128 Zhao HQ et al; Yao Xue Xue Bao 38 (4): 279-82 (2003)
... Trigonelline (N-methylnicotinic acid) /is a metabolite of nicotinamide/.
PMID:8082753 Berglund T; FEBS Lett 351 (2): 145-9 (1994)
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
97
PharmaCompass offers a list of Trigonelline API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Trigonelline manufacturer or Trigonelline supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Trigonelline manufacturer or Trigonelline supplier.
PharmaCompass also assists you with knowing the Trigonelline API Price utilized in the formulation of products. Trigonelline API Price is not always fixed or binding as the Trigonelline Price is obtained through a variety of data sources. The Trigonelline Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Trigonelline manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Trigonelline, including repackagers and relabelers. The FDA regulates Trigonelline manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Trigonelline API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Trigonelline supplier is an individual or a company that provides Trigonelline active pharmaceutical ingredient (API) or Trigonelline finished formulations upon request. The Trigonelline suppliers may include Trigonelline API manufacturers, exporters, distributors and traders.
Trigonelline Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Trigonelline GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Trigonelline GMP manufacturer or Trigonelline GMP API supplier for your needs.
A Trigonelline CoA (Certificate of Analysis) is a formal document that attests to Trigonelline's compliance with Trigonelline specifications and serves as a tool for batch-level quality control.
Trigonelline CoA mostly includes findings from lab analyses of a specific batch. For each Trigonelline CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Trigonelline may be tested according to a variety of international standards, such as European Pharmacopoeia (Trigonelline EP), Trigonelline JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Trigonelline USP).
