
Synopsis
0
KDMF
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
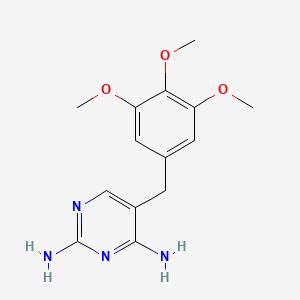

1. Proloprim
2. Trimpex
1. 738-70-5
2. Proloprim
3. Trimpex
4. Trimetoprim
5. Bactramin
6. Monotrim
7. Monotrimin
8. Trimopan
9. Wellcoprim
10. 2,4-diamino-5-(3,4,5-trimethoxybenzyl)pyrimidine
11. Monoprim
12. Syraprim
13. Trimanyl
14. Triprim
15. Uretrim
16. 5-(3,4,5-trimethoxybenzyl)pyrimidine-2,4-diamine
17. Trimethoprimum
18. Trimethoprime
19. 5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-diamine
20. Nsc-106568
21. Nih 204
22. Primsol
23. Component Of Bactrim
24. Bw 56-72
25. 2,4-pyrimidinediamine, 5-[(3,4,5-trimethoxyphenyl)methyl]-
26. Infectotrimet
27. Polytrim
28. Tcmdc-125538
29. 5-(3,4,5-trimethoxybenzyl)-2,4-pyrimidinediamine
30. Trimethoprim-d9
31. 5-[(3,4,5-trimethoxyphenyl)methyl]-2,4-pyrimidinediamine
32. Nsc 106568
33. 5-(3,4,5-trimethoxybenzyl)-2,4-diaminopyrimidine
34. Bw-56-72
35. Chebi:45924
36. Chembl22
37. Trimpex (tn)
38. 2,4-pyrimidinediamine, 5-((3,4,5-trimethoxyphenyl)methyl)-
39. Abaprim
40. Mfcd00036761
41. Apo-sulfatrim
42. Bw 5672
43. Pyrimidine, 2,4-diamino-5-(3,4,5-trimethoxybenzyl)-
44. Mls000079023
45. An164j8y0x
46. Briscotrim
47. Novotrimel
48. Streptoplus
49. Sulfoxaprim
50. Trimethioprim
51. Urobactrim
52. Wellcoprin
53. Anitrim
54. Antrima
55. Antrimox
56. Bacidal
57. Bacticel
58. Bactoprim
59. Bencole
60. Bethaprim
61. Biosulten
62. Chemotrin
63. Colizole
64. Conprim
65. Cotrimel
66. Duocide
67. Esbesul
68. Espectrin
69. Euctrim
70. Exbesul
71. Fermagex
72. Fortrim
73. Ikaprim
74. Kombinax
75. Lagatrim
76. Lastrim
77. Metoprim
78. Pancidim
79. Protrin
80. Resprim
81. Salvatrim
82. Setprin
83. Sinotrim
84. Sugaprim
85. Sulfamar
86. Sulthrim
87. Sultrex
88. Trimexol
89. Trimezol
90. Trimono
91. Trisulcom
92. Trisulfam
93. Trisural
94. Utetrin
95. Velaten
96. Xeroprim
97. Zamboprim
98. 2,4-pyrimidinediamine, 5-((3,4,5-trimethoxyphenyl)-methyl)-
99. Bacdan
100. Bacide
101. Deprim
102. Omstat
103. Purbal
104. Roubac
105. Roubal
106. Stopan
107. Toprim
108. Trisul
109. Bacin
110. Bacta
111. Futin
112. Nih-204
113. Trimpex 200
114. Co-trimoxizole
115. Lagatrim Forte
116. Septrin Forte
117. Alcorim-f
118. Colizole Ds
119. Septrin S
120. Septrin Ds
121. Smz-tmp
122. Nsc106568
123. Trimez-ifsa
124. U-prin
125. Component Of Septra
126. Ncgc00016055-05
127. Trimethopriom
128. 5-((3,4,5-trimethoxyphenyl)methyl)-2,4-pyrimidinediamine
129. Bactifor
130. Cas-738-70-5
131. Dosulfin
132. Instalac
133. Smr000035999
134. Trimetoprim [dcit]
135. Trimogal
136. Lescot
137. Tiempe
138. Trimetoprim [polish]
139. Resprim Forte
140. Trimethoprim 100 Microg/ml In Acetonitrile
141. Uro-d S
142. Dsstox_cid_3712
143. Tmp Smx
144. Dsstox_rid_77158
145. Dsstox_gsid_23712
146. 5-(3,4,5-trimethoxy-benzyl)-pyrimidine-2,4-diamine
147. Trimethoprime [inn-french]
148. Trimethoprimum [inn-latin]
149. Trimetoprima [inn-spanish]
150. 2,4-diamino-5-(3',4',5'-trimethoxybenzyl)pyrimidine
151. Bacterial [antibiotic]
152. Nih 204 (van)
153. Trimethoprim D3 (4-methoxy D3)
154. Proloprim (tn)
155. Wr 5949
156. Ccris 2410
157. Hsdb 6781
158. Sr-01000075652
159. Einecs 212-006-2
160. 5-(3, 4, 5-trimethoxybenzyl)-2, 4-pyrimidinediamine
161. Brn 0625127
162. Unii-an164j8y0x
163. Trimethoprim (jan/usp/inn)
164. 5-{[3,4,5-tris(methyloxy)phenyl]methyl}pyrimidine-2,4-diamine
165. Ai3-52594
166. Trimethoprim D3 (4-methoxy D3) 100 Microg/ml In Acetonitrile
167. B-lock
168. Kuc103659n
169. Trimethoprim,(s)
170. Prestwick_485
171. Ksc-4-158
172. Trimethoprim (tmp)
173. Bactrim (salt/mix)
174. Azt + Tmp/smx (mixture) Combination
175. Spectrum_000167
176. Tocris-0650
177. Trimethoprim [usan:usp:inn:ban:jan]
178. 2w9h
179. 3fl9
180. 3n0h
181. 3s3v
182. 4km2
183. Opera_id_1760
184. Prestwick0_000208
185. Prestwick1_000208
186. Prestwick2_000208
187. Prestwick3_000208
188. Spectrum2_000937
189. Spectrum3_000643
190. Spectrum4_000372
191. Spectrum5_001559
192. Lopac-t-7883
193. Trimethoprim [mi]
194. Trimethoprim [inn]
195. Trimethoprim [jan]
196. Epitope Id:119684
197. Upcmld-dp132
198. T 7883
199. Trimethoprim [hsdb]
200. Trimethoprim [usan]
201. Trimethoprim [vandf]
202. Lopac0_001271
203. Oprea1_495058
204. Schembl24506
205. Bspbio_000195
206. Bspbio_002245
207. Kbiogr_000863
208. Kbioss_000647
209. Trimethoprim [mart.]
210. 5-25-13-00429 (beilstein Handbook Reference)
211. Mls001201740
212. Mls002303068
213. Mls002548881
214. Bidd:gt0190
215. Divk1c_000589
216. Spectrum1500595
217. Trimethoprim [usp-rs]
218. Trimethoprim [who-dd]
219. Spbio_000874
220. Spbio_002116
221. Bpbio1_000215
222. Dtxsid3023712
223. Upcmld-dp132:001
224. Bdbm18069
225. Gtpl10931
226. Hms501n11
227. Kbio1_000589
228. Kbio2_000647
229. Kbio2_003215
230. Kbio2_005783
231. Kbio3_001465
232. Trimethoprim [green Book]
233. Trimethoprim, >=98% (hplc)
234. Ninds_000589
235. 2,4,5-trimethoxybenzyl)pyrimidine
236. Hms1568j17
237. Hms1921i03
238. Hms2090d14
239. Hms2092a10
240. Hms2095j17
241. Hms2230l06
242. Hms3259i11
243. Hms3263p04
244. Hms3371o18
245. Hms3652e03
246. Hms3712j17
247. Pharmakon1600-01500595
248. Trimethoprim [ep Impurity]
249. Trimethoprim [orange Book]
250. Trimethoprim For System Suitability
251. Trimethoprim [ep Monograph]
252. 2,4,5-trimethoxyphenzyl)pyrimidine
253. Albb-028968
254. Bcp12148
255. Cotrim Component Trimethoprim
256. Hy-b0510
257. Septra Component Trimethoprim
258. Zinc6627681
259. Co-trimoxazole Component Trimethoprim
260. Tox21_110291
261. Tox21_200157
262. Tox21_501271
263. Trimethoprim [usp Monograph]
264. Bactrim Component Trimethoprim
265. Bbl005584
266. Ccg-40335
267. Nsc752719
268. Nsc757370
269. S3129
270. Stk177322
271. Stl455117
272. Uroplus Component Trimethoprim
273. Trimethoprimum [who-ip Latin]
274. Akos001650069
275. Sulfatrim Component Trimethoprim
276. Sulmeprim Component Trimethoprim
277. Tox21_110291_1
278. Ac-8427
279. Bw-5672
280. Db00440
281. Ks-1145
282. Lp01271
283. Nc00483
284. Nsc-752719
285. Nsc-757370
286. Sdccgsbi-0051237.p004
287. Trimethoprim Component Of Cotrim
288. Trimethoprim Component Of Septra
289. Bactrim Ds Component Trimethoprim
290. Idi1_000589
291. Smp2_000262
292. Trimethoprim 100 Microg/ml In Methanol
293. Trimethoprim Component Of Bactrim
294. Trimethoprim Component Of Uroplus
295. Ncgc00016055-01
296. Ncgc00016055-02
297. Ncgc00016055-03
298. Ncgc00016055-04
299. Ncgc00016055-06
300. Ncgc00016055-07
301. Ncgc00016055-08
302. Ncgc00016055-09
303. Ncgc00016055-10
304. Ncgc00016055-11
305. Ncgc00016055-12
306. Ncgc00016055-13
307. Ncgc00016055-14
308. Ncgc00016055-16
309. Ncgc00016055-17
310. Ncgc00016055-27
311. Ncgc00024707-01
312. Ncgc00024707-03
313. Ncgc00024707-04
314. Ncgc00024707-05
315. Ncgc00024707-06
316. Ncgc00024707-07
317. Ncgc00024707-08
318. Ncgc00257711-01
319. Ncgc00261956-01
320. Cotrim D.s. Component Trimethoprim
321. Sy031734
322. Trimethoprim Component Of Sulfatrim
323. Trimethoprim Component Of Sulmeprim
324. Trimethoprim/sulfamethoxazole (commercial)
325. Sbi-0051237.p003
326. Db-055812
327. Sulfamethoprim Component Trimethoprim
328. Trimethoprim Component Of Bactrim Ds
329. 2, 5-[(3,4,5-trimethoxyphenyl)methyl]-
330. Ab00052118
331. Bb 0258034
332. Eu-0101271
333. Ft-0601630
334. Ft-0675578
335. Ft-0675579
336. Ft-0675580
337. Sw196690-3
338. T2286
339. Trimethoprim 1000 Microg/ml In Acetonitrile
340. Trimethoprim Component Of Cotrim D.s.
341. Bactrim Pediatric Component Trimethoprim
342. C01965
343. D00145
344. Trimethoprim Component Of Sulfamethoprim
345. Trimethoprim, Crystallized, >=99.0% (hplc)
346. Wln: T6n Cnj Bz Dz E1r Co1 Do1 Eo1
347. 5-(3,5-trimethoxybenzyl)-2,4-diaminopyrimidine
348. Ab00052118-30
349. Ab00052118-32
350. Ab00052118_33
351. Ab00052118_34
352. Trimethoprim, Vetranal(tm), Analytical Standard
353. 738t705
354. Formulated Trimethoprim (nsc 106568) In Ethanol
355. Q422665
356. Trimethoprim Component Of Bactrim Pediatric
357. 2,4-diamino-5-(3,4,5-trimethoxybenzyl) Pyrimidine
358. 2,4-diamino-5-(3,4,5-trimethoxybenzyl)-pyrimidine
359. Pyrimidine,4-diamino-5-(3,4,5-trimethoxybenzyl)-
360. Sr-01000075652-1
361. Sr-01000075652-3
362. Sr-01000075652-6
363. W-104441
364. 5-(3,4,5-trimethoxybenzyl)-2,4-pyrimidinediamine #
365. Brd-k07208025-001-06-5
366. Sr-01000075652-10
367. 2-amino-5-(3,4,5-trimethoxybenzyl)-4-pyrimidinylamine
368. F0914-5266
369. Trimethoprim, Certified Reference Material, Tracecert(r)
370. Z1522566629
371. 5-(3,4,5-trimethoxybenzyl)pyrimidine-2,4(1h,3h)-diimine
372. Trimethoprim, British Pharmacopoeia (bp) Reference Standard
373. Trimethoprim, European Pharmacopoeia (ep) Reference Standard
374. Trimethoprim, United States Pharmacopeia (usp) Reference Standard
375. Trimethoprim For System Suitability, European Pharmacopoeia (ep) Reference Standard
376. Trimethoprim, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 290.32 g/mol |
|---|---|
| Molecular Formula | C14H18N4O3 |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 290.13789045 g/mol |
| Monoisotopic Mass | 290.13789045 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 307 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Polytrim |
| PubMed Health | Trimethoprim (By mouth) |
| Drug Classes | Antibiotic, Antiseptic |
| Active Ingredient | trimethoprim sulfate; Polymyxin b sulfate |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 10,000 units/ml; eq 1mg base/ml |
| Market Status | Prescription |
| Company | Allergan |
| 2 of 6 | |
|---|---|
| Drug Name | Primsol |
| PubMed Health | Trimethoprim (By mouth) |
| Drug Classes | Antibiotic, Antiseptic |
| Active Ingredient | Trimethoprim hydrochloride |
| Dosage Form | Solution |
| Route | Oral |
| Strength | eq 50mg base/5ml |
| Market Status | Prescription |
| Company | Fsc |
| 3 of 6 | |
|---|---|
| Drug Name | Trimethoprim |
| Drug Label | Trimethoprim is a synthetic antibacterial available as 100 mg tablets for oral administration.Trimethoprim is 2,4-Diamino-5-(3,4,5-trimethoxybenzyl) pyrimidine. It is a white to cream colored, odorless, bitter compound. The structural formula is repr... |
| Active Ingredient | Trimethoprim |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg |
| Market Status | Prescription |
| Company | Teva; Novel Labs; Watson Labs |
| 4 of 6 | |
|---|---|
| Drug Name | Polytrim |
| PubMed Health | Trimethoprim (By mouth) |
| Drug Classes | Antibiotic, Antiseptic |
| Active Ingredient | trimethoprim sulfate; Polymyxin b sulfate |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 10,000 units/ml; eq 1mg base/ml |
| Market Status | Prescription |
| Company | Allergan |
| 5 of 6 | |
|---|---|
| Drug Name | Primsol |
| PubMed Health | Trimethoprim (By mouth) |
| Drug Classes | Antibiotic, Antiseptic |
| Active Ingredient | Trimethoprim hydrochloride |
| Dosage Form | Solution |
| Route | Oral |
| Strength | eq 50mg base/5ml |
| Market Status | Prescription |
| Company | Fsc |
| 6 of 6 | |
|---|---|
| Drug Name | Trimethoprim |
| Drug Label | Trimethoprim is a synthetic antibacterial available as 100 mg tablets for oral administration.Trimethoprim is 2,4-Diamino-5-(3,4,5-trimethoxybenzyl) pyrimidine. It is a white to cream colored, odorless, bitter compound. The structural formula is repr... |
| Active Ingredient | Trimethoprim |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg |
| Market Status | Prescription |
| Company | Teva; Novel Labs; Watson Labs |
Anti-Infective Agents, Urinary; Antimalarials; Antimetabolites; Folic Acid Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Trimethoprim given alone has also been effective for urinary tract infections, but the development of resistant organisms limits the usefulness of this treatment.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1056
Trimethoprim is indicated in the treatment of initial, uncomplicated urinary tract infections caused by susceptible strains of Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Enterobacter species, & coagulase-negative Staphylococcus species, including Staphylococcus saprophyticus. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2848
trimethoprim is used in the prophylaxis of bacterial urinary tract infections. /NOT included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2848
For more Therapeutic Uses (Complete) data for TRIMETHOPRIM (8 total), please visit the HSDB record page.
Because trimethoprim may interfere with folic acid metabolism, the drug should be used with caution in nursing women.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 842
Adverse GI reactions occur in approximately 6% of patients receiving trimethoprim and may include epigastric discomfort, nausea, vomiting, glossitis, and abnormal taste sensation. Elevations in serum aminotransferase (transaminase) and bilirubin concentrations have been reported in patients receiving the drug, but the clinical importance of these findings is not known. Cholestatic jaundice has been reported rarely.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 842
The most frequent adverse effects of trimethoprim are rash and pruritus. Mild to moderate rashes appearing 7-14 days after initiation of trimethoprim therapy reportedly occur in 2.9-6.7% of patients receiving 200 mg of the drug daily. Rashes are generally maculopapular, morbilliform, and pruritic. Rashes have been reported to occur in up to 24% of patients receiving 400 mg or more trimethoprim for 14 days. Photosensitivity (e.g., erythematous phototoxic eruptions with subsequent hyperpigmentation of sun-exposed skin) also has occurred. Exfoliative dermatitis, toxic epidermal necrolysis (Lyell's syndrome), erythema multiforme, and Stevens-Johnson syndrome have been reported rarely in patients receiving the drug. Anaphylaxis also has occurred rarely.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 842
Safety and efficacy of trimethoprim in infants younger than 2 months of age and efficacy of the drug when used as single agent in children younger than 12 years of age have not been established. Trimethoprim should be used with caution in children who have the fragile X chromosome associated with mental retardation, because folate depletion may worsen the psychomotor regression associated with the disorder.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 842
For more Drug Warnings (Complete) data for TRIMETHOPRIM (28 total), please visit the HSDB record page.
As a monotherapy, trimethoprim is indicated for the treatment of acute episodes of uncomplicated urinary tract infections caused by susceptible bacteria, including _E. coli._, _K. pneumoniae_, _Enterobacter spp._, _P. mirabilis_, and coagulase-negative _Staphylococcus_ species. In various formulations in combination with [sulfamethoxazole], trimethoprim is indicated for the following infections caused by bacteria with documented susceptibility: urinary tract infections, acute otitis media in pediatric patients (when clinically indicated), acute exacerbations of chronic bronchitis in adults, enteritis caused by susceptible _Shigella_, prophylaxis and treatment of _Pneumocystis jiroveci_ pneumonia, and travelers' diarrhea caused by enterotoxigenic _E. coli_. Trimethoprim is available as an ophthalmic solution in combination with [polymyxin B] for the treatment of acute bacterial conjunctivitis, blepharitis, and blepharoconjunctivitis caused by susceptible bacteria.
FDA Label
Trimethoprim exerts its antimicrobial effects by inhibiting an essential step in the synthesis of bacterial nucleic acids and proteins. It has shown activity against several species of gram-negative bacteria, as well as coagulase-negative _Staphylococcus_ species. Resistance to trimethoprim may arise via a variety of mechanisms, including alterations to the bacterial cell wall, overproduction of dihydrofolate reductase, or production of resistant dihydrofolate reductase. Rarely, trimethoprim can precipitate the development of blood disorders (e.g. thrombocytopenia, leukopenia, etc.) which may be preceded by symptoms such as sore throat, fever, pallor, and or purpura - patients should be monitored closely for the development of these symptoms throught the course of therapy. As antimicrobial susceptibility patterns are geographically distinct, local antibiograms should be consulted to ensure adequate coverage of relevant pathogens prior to use.
Cytochrome P-450 CYP2C8 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP2C8. (See all compounds classified as Cytochrome P-450 CYP2C8 Inhibitors.)
Anti-Infective Agents, Urinary
Substances capable of killing agents causing urinary tract infections or of preventing them from spreading. (See all compounds classified as Anti-Infective Agents, Urinary.)
Folic Acid Antagonists
Inhibitors of the enzyme, dihydrofolate reductase (TETRAHYDROFOLATE DEHYDROGENASE), which converts dihydrofolate (FH2) to tetrahydrofolate (FH4). They are frequently used in cancer chemotherapy. (From AMA, Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Folic Acid Antagonists.)
Antimalarials
Agents used in the treatment of malaria. They are usually classified on the basis of their action against plasmodia at different stages in their life cycle in the human. (From AMA, Drug Evaluations Annual, 1992, p1585) (See all compounds classified as Antimalarials.)
Anti-Dyskinesia Agents
Drugs used in the treatment of movement disorders. Most of these act centrally on dopaminergic or cholinergic systems. Among the most important clinically are those used for the treatment of Parkinson disease (ANTIPARKINSON AGENTS) and those for the tardive dyskinesias. (See all compounds classified as Anti-Dyskinesia Agents.)
J01EE01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01E - Sulfonamides and trimethoprim
J01EA - Trimethoprim and derivatives
J01EA01 - Trimethoprim
Absorption
Steady-state concentrations are achieved after approximately 3 days of repeat administration. Average peak serum concentrations of approximately 1 g/mL (Cmax) are achieved within 1 to 4 hours (Tmax) following the administration of a single 100mg dose. Trimethoprim appears to follow first-order pharmacokinetics, as a single 200mg dose results in serum concentrations approximately double that of a 100mg dose. The steady-state AUC of orally administered trimethoprim is approximately 30 mg/Lh.
Route of Elimination
Approximately 10-20% of an ingested trimethoprim dose is metabolized, primarily in the liver, while a large portion of the remainder is excreted unchanged in the urine. Following oral administration, 50% to 60% of trimethoprim is excreted in the urine within 24 hours, approximately 80% of which is unchanged parent drug.
Volume of Distribution
Trimethoprim is extensively distributed into various tissues following oral administration. It distributes well into sputum, middle ear fluid, and bronchial secretions. Trimethoprim distributes efficiently into vaginal fluids, with observed concentrations approximately 1.6-fold higher than those seen in the serum. It may pass the placental barrier and into breast milk. Trimethoprim is also sufficiently excreted in the feces to markedly reduce and/or eliminate trimethoprim-susceptible fecal flora.
Clearance
Following oral administration, the renal clearance of trimethoprim has been variably reported between 51.7 - 91.3 mL/min.
Trimethoprim is widely distributed into body tissues & fluids including the aqueous humor, middle ear fluid, saliva, lung tissue, sputum, seminal fluid, prostatic tissue & fluid, vaginal secretions, bile, bone, & /cerebrospinal fluid/. The apparent volume of distribution of trimethoprim in adults with normal renal function ranges from 100-120 l. ... Trimethoprim is 42-46% bound to plasma proteins. Trimethoprim readily crosses the placenta, & amniotic fluid concns are reported to be 80% of concurrent maternal serum concns.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 843
Only small amounts of trimethoprim are excreted in feces via biliary elimination. Trimethoprim may be moderately removed by hemodialysis.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 844
Trimethoprim is readily & almost completely absorbed from the GI tract. Peak serum concns of approx 1, 1.6, & 2 ug/ml are reached in 1-4 hr after single 100-, 160-, & 200 mg oral doses of trimethoprim. Following multiple-dose oral admin, steady-state peak serum concns of trimethoprim usually are 50% greater than those obtained after single-dose admin of the drug. Steady-state serum concns range from 1.2-3.2 ug/ml following oral admin of 160 mg of trimethoprim every 12 hr in adults with renal function.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 843
Rapidly and widely distributed to various tissues and fluids, including kidneys, liver, spleen, bronchial secretions, saliva, and seminal fluid. Trimethoprim has also been demonstrated in bile; aqueous humor; bone marrow and spongy, but not compact, bone.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2849
For more Absorption, Distribution and Excretion (Complete) data for TRIMETHOPRIM (12 total), please visit the HSDB record page.
Trimethoprim undergoes oxidative metabolism to a number of metabolites, the most abundant of which are the demethylated 3'- and 4'- metabolites, accounting for approximately 65% and 25% of the total metabolite formation, respectively. Minor products include N-oxide metabolites (<5%) and benzylic metabolites in even smaller quantities. The parent drug is considered to be the therapeutically active form. The majority of trimethoprim biotransformation appears to involve CYP2C9 and CYP3A4 enzymes, with CYP1A2 contributing to a lesser extent.
Trimethoprim is metabolized in the liver to oxide and hydroxylated metabolites ... .
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 844
The pharmacokinetics were studied of sulfadimethoxine (SDM) or sulfamethoxazole (SMX) in combination with trimethoprim (TMP) administered as a single oral dose (25 mg + 5 mg/kg bw) to 2 groups of 6 healthy pigs. The elimination half-lives of SMX & TMP were quite similar (2-3 hr); SDM had a relatively long half-life of 13 hr. Both sulfonamides (S) were exclusively metabolized to N4-acetyl derivatives but to different extents. The main metabolic pathway for TMP was O-demethylation & subsequent conjugation. In addition, the plasma concns of these drugs & their main metabolites after medication with different in-feed concns were determined. The drug (S:TMP) concns in the feed were 250:50, 500:100, & 1000:200 mg/kg. Steady-state concns were achieved within 48 hr of feed medication, twice daily (SDM+TMP) or 3 times/day (SMX+TMP). Protein binding of SDM & its metabolite was high (>93%), whereas SMX, TMP & their metabolites showed moderate binding (48-75%). Feed medication with 500 ppm sulfonamide combined with 100 ppm TMP provided minimum steady-state plasma concns (C(ss,min)) higher than the concn required for inhibition of the growth of 90% of Actinobacillus pleuropneumoniae strains (n=20).
Mengelers MF, et al; Vet Res Commun 25 (6): 461-481. 2001.
Trimethoprim half-life ranges from 8-10 hours, but may be prolonged in patients with renal dysfunction.
Trimethoprim has a serum half-life of approx 8-11 hr in adults with normal renal function. In adults with creatinine clearances of 10-30 or 0-10 ml/min, serum half-life of the drug may incr to 15 hr or >26 hr, respectively. Trimethoprim serum half-lives of about 7.7 & 5.5 hr have been reported in children <1 yr of age & between 1 & 10 yr of age, respectively.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 843
Trimethoprim is a reversible inhibitor of dihydrofolate reductase, one of the principal enzymes catalyzing the formation of tetrahydrofolic acid (THF) from dihydrofolic acid (DHF). Tetrahydrofolic acid is necessary for the biosynthesis of bacterial nucleic acids and proteins and ultimately for continued bacterial survival - inhibiting its synthesis, then, results in bactericidal activity. Trimethoprim binds with a much stronger affinity to bacterial dihydrofolate reductase as compared to its mammalian counterpart, allowing trimethoprim to selectively interfere with bacterial biosynthetic processes. Trimethoprim is often given in combination with sulfamethoxazole, which inhibits the preceding step in bacterial protein synthesis - given together, sulfamethoxazole and trimethoprim inhibit two consecutive steps in the biosynthesis of bacterial nucleic acids and proteins. As a monotherapy trimethoprim is considered bacteriostatic, but in combination with sulfamethoxazole is thought to exert bactericidal activity.
Trimethoprim is a bacteriostatic lipophilic weak base structurally related to pyrimethamine. It binds to and reversibly inhibits the bacterial enzyme dihydrofolate reductase, selectively blocking conversion of dihydrofolic acid to its functional form, tetrahydrofolic acid. This depletes folate, an essential cofactor in the biosynthesis of nucleic acids, resulting in interference with bacterial nucleic acid and protein production. Bacterial dihydrofolate reductase is approximately 50,000 to 60,000 times more tightly bound by trimethoprim than is the corresponding mammalian enzyme.
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, V.IB p.2531 (1992)
To determine the incidence & severity of hyperkalemia during trimethoprim therapy, 30 consecutive patients with acquired immunodeficiency syndrome receiving high-dose (20 mg/kg/day) trimethoprim were studied; in addition, the mechanism of trimethoprim-induced hyperkalemia was investigated in rats. Trimethoprim increased serum potassium concn by 0.6 mmol/l despite normal adrenocortical function & glomerular filtration rate. Serum potassium levels >5 mmol/l were observed during trimethoprim treatment in 15 of 30 patients. In rats, iv trimethoprim inhibited renal potassium excretion by 40% & increased sodium excretion by 46%. It was concluded that trimethoprim blocks apical membrane sodium channels in the mammalian distal nephron. As a consequence, the transepithelial voltage is reduced & potassium secretion is inhibited. Decreased renal potassium excretion secondary to these direct effects on kidney tubules leads to hyperkalemia in a substantial number of patients being treated with trimethoprim-containing drugs.
PMID:8328738 Velazquez H, et al; Ann. Intern. Med. 119 (Aug 15): 296-301 (1993)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
31
PharmaCompass offers a list of Trimethoprim API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Trimethoprim manufacturer or Trimethoprim supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Trimethoprim manufacturer or Trimethoprim supplier.
PharmaCompass also assists you with knowing the Trimethoprim API Price utilized in the formulation of products. Trimethoprim API Price is not always fixed or binding as the Trimethoprim Price is obtained through a variety of data sources. The Trimethoprim Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Trimethoprim manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Trimethoprim, including repackagers and relabelers. The FDA regulates Trimethoprim manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Trimethoprim API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Trimethoprim manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Trimethoprim supplier is an individual or a company that provides Trimethoprim active pharmaceutical ingredient (API) or Trimethoprim finished formulations upon request. The Trimethoprim suppliers may include Trimethoprim API manufacturers, exporters, distributors and traders.
click here to find a list of Trimethoprim suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Trimethoprim DMF (Drug Master File) is a document detailing the whole manufacturing process of Trimethoprim active pharmaceutical ingredient (API) in detail. Different forms of Trimethoprim DMFs exist exist since differing nations have different regulations, such as Trimethoprim USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Trimethoprim DMF submitted to regulatory agencies in the US is known as a USDMF. Trimethoprim USDMF includes data on Trimethoprim's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Trimethoprim USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Trimethoprim suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Trimethoprim Drug Master File in Japan (Trimethoprim JDMF) empowers Trimethoprim API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Trimethoprim JDMF during the approval evaluation for pharmaceutical products. At the time of Trimethoprim JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Trimethoprim suppliers with JDMF on PharmaCompass.
A Trimethoprim CEP of the European Pharmacopoeia monograph is often referred to as a Trimethoprim Certificate of Suitability (COS). The purpose of a Trimethoprim CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Trimethoprim EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Trimethoprim to their clients by showing that a Trimethoprim CEP has been issued for it. The manufacturer submits a Trimethoprim CEP (COS) as part of the market authorization procedure, and it takes on the role of a Trimethoprim CEP holder for the record. Additionally, the data presented in the Trimethoprim CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Trimethoprim DMF.
A Trimethoprim CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Trimethoprim CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Trimethoprim suppliers with CEP (COS) on PharmaCompass.
A Trimethoprim written confirmation (Trimethoprim WC) is an official document issued by a regulatory agency to a Trimethoprim manufacturer, verifying that the manufacturing facility of a Trimethoprim active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Trimethoprim APIs or Trimethoprim finished pharmaceutical products to another nation, regulatory agencies frequently require a Trimethoprim WC (written confirmation) as part of the regulatory process.
click here to find a list of Trimethoprim suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Trimethoprim as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Trimethoprim API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Trimethoprim as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Trimethoprim and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Trimethoprim NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Trimethoprim suppliers with NDC on PharmaCompass.
Trimethoprim Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Trimethoprim GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Trimethoprim GMP manufacturer or Trimethoprim GMP API supplier for your needs.
A Trimethoprim CoA (Certificate of Analysis) is a formal document that attests to Trimethoprim's compliance with Trimethoprim specifications and serves as a tool for batch-level quality control.
Trimethoprim CoA mostly includes findings from lab analyses of a specific batch. For each Trimethoprim CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Trimethoprim may be tested according to a variety of international standards, such as European Pharmacopoeia (Trimethoprim EP), Trimethoprim JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Trimethoprim USP).
