
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Ci 898
2. Ci-898
3. Ci898
4. Hydrate, Trimetrexate
5. Jb 11
6. Jb-11
7. Jb11
8. Monohydrate, Monoacetate Trimetrexate
9. Nsc 249008
10. Nsc 328564
11. Nsc-249008
12. Nsc-328564
13. Nsc249008
14. Nsc328564
15. Trimetrexate Hydrate
16. Trimetrexate Monohydrate, Monoacetate
1. 52128-35-5
2. Ci-898
3. Jb-11
4. Trimetrexatum [inn-latin]
5. Trimetrexato [inn-spanish]
6. Nsc-249008
7. Neutrexin
8. Tmq
9. Nsc 249008
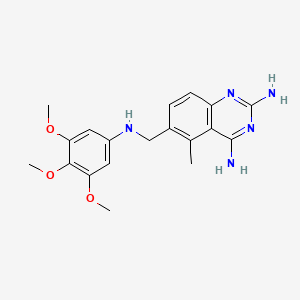
10. 5-methyl-6-[(3,4,5-trimethoxyanilino)methyl]quinazoline-2,4-diamine
11. Nsc249008
12. Upn4iti8t4
13. Chembl119
14. 2,4-diamino-5-methyl-6-((3,4,5-trimethoxyanilino)methyl)quinazoline
15. 2,4-quinazolinediamine, 5-methyl-6-(((3,4,5-trimethoxyphenyl)amino)methyl)-
16. 5-methyl-6-(((3,4,5-trimethoxyphenyl)amino)methyl)quinazoline-2,4-diamine
17. Trimetrexato
18. Trimetrexatum
19. 5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}quinazoline-2,4-diamine
20. Trimetrexate Hydrate
21. Jb 11
22. 2,4-quinazolinediamine,5-methyl-6-[[(3,4,5-trimethoxyphenyl)amino]methyl]-
23. Hsdb 6545
24. Ncgc00161419-02
25. Unii-upn4iti8t4
26. Trimetrexate (usan/inn)
27. Trimetrexate [usan:inn:ban]
28. 5-methyl-6-[[(3,4,5-trimethoxyphenyl)amino]methyl]quinazoline-2,4-diamine
29. 5-methyl-6-(((3,4,5-trimethoxyphenyl)amino)methyl)-2,4-quinazolinediamine
30. Trimetrexate [mi]
31. Trimetrexate [inn]
32. Trimetrexate [hsdb]
33. Trimetrexate [usan]
34. Schembl3983
35. Trimetrexate [vandf]
36. Bidd:gt0129
37. Trimetrexate [who-dd]
38. Chebi:9737
39. Gtpl7613
40. Dtxsid3023714
41. Bdbm18268
42. Zinc598852
43. Bcp15515
44. 6-[((3,4,5-trimethoxyphenyl)amino)methyl]-5-methyl-2,4-quinazolinediamine
45. Db01157
46. 5-methyl-6-({[3,4,5-tris(methyloxy)phenyl]amino}methyl)quinazoline-2,4-diamine
47. Ncgc00161419-01
48. Ncgc00161419-03
49. Ncgc00161419-04
50. Hy-10373
51. Ft-0649497
52. D06238
53. A913386
54. Q7842225
55. 2, 5-methyl-6-[[(3,4,5-trimethoxyphenyl)amino]methyl]-
56. 2,4-diamino-5-methyl-6-[(3,4,5-trimethoxyphenylamino)methyl]quinazoline
57. 251301-18-5
| Molecular Weight | 369.4 g/mol |
|---|---|
| Molecular Formula | C19H23N5O3 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 6 |
| Exact Mass | 369.18008961 g/mol |
| Monoisotopic Mass | 369.18008961 g/mol |
| Topological Polar Surface Area | 118 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 457 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antifungal Agents; Antimetabolites; Antimetabolites, Antineoplastic; Folic Acid Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Antineoplastic
Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 1529
Trimetrexate glucuronate is an investigational drug that is available for treatment use ... in a hospital setting in qualifying patients with Pneumocystis carinii pneumonia and who have exhibited serious ... intolerance to both co-trimoxazole and pentamidine. /Trimetrexate glucuronate/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 2395
Even though trimetrexate does not compete with the folate transport system for entry into cells, utilization of folates is reduced due to inhibition of dihydrofolate reductase. When trimetrexate is administered in high doses for the treatment of pneumocystis carinii pneumonia, leucovorin must be given concurrently with trimetrexate to reduce toxic effects on human tissues. It has been postulated that the absence of a folate transport system in pneumocystis carinii provides the opportunity for differential rescue of host tissue affecting antiprotozoal action.
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, V.I p.V/56 (1992)
Patients receiving trimetrexate should have frequent laboratory monitoring of hepatic and hematologic prameters, including serum alanine aminotransferase, serum aspartate aminotransferase, bilirubin, alkaline phosphatase, platelet count, and total and differential leukocyte counts. Although uncommon in AIDS patients receiving trimetrexate for pneumocystis carinii pneumonia, slight elevations of blood urea nitrogen and/or serum creatinine have been reported in patients receiving the drug for various cancers.
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, V.I p.V/56 (1992)
Isolated perfused rat liver has been used to study potential drug interactions with trimetrexate metabolism in vitro. Cimetidine, which inhibits oxidative drug metabolizing enzymes, decreased the clearance of trimetrexate to approximately one half of control values. Whether this occurs to a significant extent in vivo is unknown.
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, V.I p.V/56 (1992)
For use, with concurrent leucovorin administration (leucovorin protection), as an alternative therapy for the treatment of moderate-to-severe Pneumocystis carinii pneumonia (PCP) in immunocompromised patients, including patients with the acquired immunodeficiency syndrome (AIDS). Also used to treat several types of cancer including colon cancer.
FDA Label
Trimetrexate, a non-classical folate antagonist, is a synthetic inhibitor of the enzyme dihydrofolate reductase (DHFR). During DNA synthesis and cellular reproduction, folic acid is reduced to tetrahydrofolic acid by the enzyme folic acid reductase. By interfering with the reduction of folic acid, trimetrexate interferes with tissue cell reproduction. Generally, the most sensitive cells to the antimetabolite effect of trimetrexate are those cells which are most actively proliferating such as malignant cells, dermal epithelium, buccal and intestinal mucosa, bone marrow, fetal cells, and cells of the urinary bladder. Because the proliferation of cells in malignant tissues is greater than in most normal tissues, trimetrexate may impair the growth of the malignant tissues without causing irreversible damage to normal tissues. Due to very serious and potentially life-threatening side-effects of this drug, leucovorin must be co-administered for at least 72 hours after the last dose.
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
Antimetabolites, Antineoplastic
Antimetabolites that are useful in cancer chemotherapy. (See all compounds classified as Antimetabolites, Antineoplastic.)
Folic Acid Antagonists
Inhibitors of the enzyme, dihydrofolate reductase (TETRAHYDROFOLATE DEHYDROGENASE), which converts dihydrofolate (FH2) to tetrahydrofolate (FH4). They are frequently used in cancer chemotherapy. (From AMA, Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Folic Acid Antagonists.)
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01A - Agents against amoebiasis and other protozoal diseases
P01AX - Other agents against amoebiasis and other protozoal diseases
P01AX07 - Trimetrexate
Route of Elimination
Ten to 30% of the administered dose is excreted unchanged in the urine.
Volume of Distribution
20 8 L/m2
36.9 6 L/m2 [cancer patients]
Clearance
38 +/- 15 mL/min/m2 [patients with acquired immunodeficiency syndrome (AIDS) who had Pneumocystis carinii pneumonia (4 patients) or toxoplasmosis (2 patients). Trimetrexate was administered intravenously as a bolus injection at a dose of 30 mg/m2/day along with leucovorin 20 mg/m2 every 6 hours for 21 days]
53 +/- 41 mL/min/m2 [Cancer patients with advanced solid tumors using various dosage regimensreceiving a single-dose administration of 10 to 130 mg/m2]
30 +/- 8 mL/min/m2 [Cancer patients with advanced solid tumors using various dosage regimensafter a five-day infusion]
Clinical pharmacokinetic studies in cancer patients show that trimetrexate plasma concentration-time curves are biphasic or triphasic in form. The terminal elimination half-life averages 13.6 hr. Mean total plasma clearance and volume of distribution as steady-state values were 27.9 ml/min/sq m and 21.1 l/sq m, respectively. Cerebrospinal fluid concentration was 3.4% of that in plasma, which shows that trimetrexate does not cross the blood-brain barrier well. Trimetrexate is 86 to 94% bound to plasma proteins.
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, V.I p.V/56 (1992)
Mean oral bioavailability of the parenteral solution (glucuronate) in AIDS patients was 42%, with a mean maximum plasma concentration of 1182 ng/ml (3.2 umole/l) achieved 1.8 hr postdose. /Trimetrexate glucuromate/
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, V.I p.V/56 (1992)
Hepatic. Preclinical data strongly suggest that the major metabolic pathway is oxidative O-demethylation, followed by conjugation to either glucuronide or the sulfate.
Trimetrexate is highly metabolized by the liver. At least 2 metabolites are excreted in urine. One metabolite has been identified a 4'-O-glucuronide conjugate of trimetrexate, which is formed as a result of oxidative O-dimethylation at the 4'-position and subsequent conjugation with glucuronic acid. About 15% of a dose is excreted in urine as unchanged trimetrexate, while another 20% apparently excreted as metabolites.
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, V.I p.V/56 (1992)
11 to 20 hours
The terminal elimination half-life averages 13.6 hours.
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, V.I p.V/56 (1992)
In vitro studies have shown that trimetrexate is a competitive inhibitor of dihydrofolate reductase (DHFR) from bacterial, protozoan, and mammalian sources. DHFR catalyzes the reduction of intracellular dihydrofolate to the active coenzyme tetrahydrofolate. Inhibition of DHFR results in the depletion of this coenzyme, leading directly to interference with thymidylate biosynthesis, as well as inhibition of folate-dependent formyltransferases, and indirectly to inhibition of p.r.n. biosynthesis. The end result is disruption of DNA, RNA, and protein synthesis, with consequent cell death.
Trimetrexate binds to dihydrofolate reductase and prevents the conversion of dihydrofolate to biologically active tetrahydrofolate. It inhibits nucleic acid synthesis as a result of antithymidylate and antipurine effects.
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, V.I p.V/56 (1992)
ABOUT THIS PAGE
58
PharmaCompass offers a list of Trimetrexate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Trimetrexate manufacturer or Trimetrexate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Trimetrexate manufacturer or Trimetrexate supplier.
PharmaCompass also assists you with knowing the Trimetrexate API Price utilized in the formulation of products. Trimetrexate API Price is not always fixed or binding as the Trimetrexate Price is obtained through a variety of data sources. The Trimetrexate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Trimetrexate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Trimetrexate, including repackagers and relabelers. The FDA regulates Trimetrexate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Trimetrexate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Trimetrexate supplier is an individual or a company that provides Trimetrexate active pharmaceutical ingredient (API) or Trimetrexate finished formulations upon request. The Trimetrexate suppliers may include Trimetrexate API manufacturers, exporters, distributors and traders.
click here to find a list of Trimetrexate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Trimetrexate DMF (Drug Master File) is a document detailing the whole manufacturing process of Trimetrexate active pharmaceutical ingredient (API) in detail. Different forms of Trimetrexate DMFs exist exist since differing nations have different regulations, such as Trimetrexate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Trimetrexate DMF submitted to regulatory agencies in the US is known as a USDMF. Trimetrexate USDMF includes data on Trimetrexate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Trimetrexate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Trimetrexate suppliers with USDMF on PharmaCompass.
Trimetrexate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Trimetrexate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Trimetrexate GMP manufacturer or Trimetrexate GMP API supplier for your needs.
A Trimetrexate CoA (Certificate of Analysis) is a formal document that attests to Trimetrexate's compliance with Trimetrexate specifications and serves as a tool for batch-level quality control.
Trimetrexate CoA mostly includes findings from lab analyses of a specific batch. For each Trimetrexate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Trimetrexate may be tested according to a variety of international standards, such as European Pharmacopoeia (Trimetrexate EP), Trimetrexate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Trimetrexate USP).
