
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
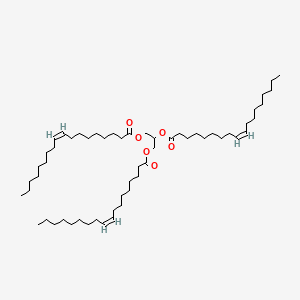

1. Glycerol Trioleate
2. Glycerol, Trioleyl
3. Trielaidin
4. Trioleate Glycerin
5. Trioleate, Glycerol
6. Trioleate-glycerin
7. Trioleoylglycerol
8. Trioleyl Glycerol
1. Glyceryl Trioleate
2. Glycerol Trioleate
3. 122-32-7
4. Glycerol Triolein
5. Oleic Triglyceride
6. Olein
7. Trioleoylglycerol
8. Oleic Acid Triglyceride
9. Trioleoylglyceride
10. Glycerin Trioleate
11. Oleyl Triglyceride
12. Raoline
13. Glyceryl-1,2,3-trioleate
14. Aldo To
15. Emery 2423
16. Olein, Tri-
17. Emery Oleic Acid Ester 2230
18. Glycerol, Tri(cis-9-octadecenoate)
19. 1,2,3-propanetriyl Trioleate
20. Hsdb 5594
21. Triglyceride Ooo
22. Edenor Nhti-g
23. Kaolube 190
24. Sn-glyceryl Trioleate
25. 1,2,3-tri(cis-9-octadecenoyl)glycerol
26. 1,2,3-tri-(9z-octadecenoyl)-glycerol
27. Actor Lo 1
28. Kemester 1000
29. Emerest 2423
30. 9-octadecenoic Acid (z)-, 1,2,3-propanetriyl Ester
31. 9-octadecenoic Acid (9z)-, 1,2,3-propanetriyl Ester
32. Estol 1433
33. Radia 7363
34. 1,2,3-tri-oleoyl-glycerol
35. 1,2,3-propanetriol Tri(9-octandecenoate)
36. Chebi:53753
37. Tg(18:1(9z)/18:1(9z)/18:1(9z))
38. 2,3-bis[[(z)-octadec-9-enoyl]oxy]propyl (z)-octadec-9-enoate
39. 9-octadecenoic Acid, 1,2,3-propanetriyl Ester
40. O05ec62663
41. Propane-1,2,3-triyl (9z,9'z,9''z)tris-octadec-9-enoate
42. Glycerine Trioleate
43. (9z)9-octadecenoic Acid 1,2,3-propanetriyl Ester
44. 1,3-bis[(9z)-octadec-9-enoyloxy]propan-2-yl (9z)-octadec-9-enoate
45. Tg 54:3
46. Einecs 204-534-7
47. Ccris 8687
48. Tri-olein
49. Unii-o05ec62663
50. 9-octadecenoic-9,10-t2 Acid, 1,2,3-propanetriyl Ester, (z,z,z)- (9ci)
51. Mfcd00137563
52. Triolein C18:1
53. Triolein, Tech Grade
54. Glyceryltrioleate
55. Triolein [inci]
56. Triolein [mi]
57. Tri(cis-9-octadecenoate)
58. Epitope Id:117714
59. Triolein [usp-rs]
60. Triolein [who-dd]
61. 1,2,3-propanetriyl Ester
62. Ec 204-534-7
63. Glyceryl Trioleate, ~65%
64. Schembl23730
65. Glyceryl Trioleate, >=99%
66. 9-octadecenoic Acid (9z)-, 1,1',1''-(1,2,3-propanetriyl) Ester
67. Glyceryl Trioleate [ii]
68. Chembl4297656
69. Dtxsid3026988
70. Glyceryl Trioleate [hsdb]
71. Glyceryl Trioleate [vandf]
72. Glyceryl Trioleate [mart.]
73. Glyceryl Trioleate [who-dd]
74. Hy-n1981
75. Triolein, [9,10-3h(n)]-
76. Lmgl03010250
77. S3590
78. Zinc85545180
79. Akos024437536
80. Db13038
81. Glyceryl Trioleate, >=97.0% (tlc)
82. 1,2,3-tri-(9z-octadecenoyl)-sn-glycerol
83. Cs-0018302
84. G0089
85. Glyceryl Trioleate, Technical, >=60% (gc)
86. (z)-1,2,3-propanetriyl Ester 9-octadecenoate
87. (9z)-1,2,3-propanetriyl Ester 9-octadecenoate
88. Glyceryl Trioleate, Analytical Reference Material
89. Q413929
90. (9z)-1,2,3-propanetriyl Ester 9-octadecenoic Acid
91. (z)-1,2,3-propanetriyl Ester 9-octadecenoic Acid
92. (z)-9-octadecenoic Acid, 1,2,3-propanetriyl Ester
93. J-004788
94. Propane-1,2,3-triyl Tris[(9z)-octadec-9-enoate]
95. Ac7b54b8-0e34-455f-a1e0-442f3ecd69ea
96. Triolein, European Pharmacopoeia (ep) Reference Standard
97. Tg(18:1(9z)/18:1(9z)/18:1(9z))[iso]
98. (9z)-1,1',1''-(1,2,3-propanetriyl) Ester 9-octadecenoate
99. (9z)-1,1',1''-(1,2,3-propanetriyl) Ester 9-octadecenoic Acid
100. 9-octadecenoic Acid, 1,2,3-propanetriyl Ester, (9z,9'z,9''z)-
101. Triolein (18:1 Tg), 1,2,3-tri-(9z-octadecenoyl)-glycerol, Neat Oil
| Molecular Weight | 885.4 g/mol |
|---|---|
| Molecular Formula | C57H104O6 |
| XLogP3 | 22.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 53 |
| Exact Mass | 884.78329103 g/mol |
| Monoisotopic Mass | 884.78329103 g/mol |
| Topological Polar Surface Area | 78.9 Ų |
| Heavy Atom Count | 63 |
| Formal Charge | 0 |
| Complexity | 1010 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 3 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/The aim of this study was/ To identify asymptomatic boys with X-linked adrenoleukodystrophy who have a normal magnetic resonance image (MRI), and to assess the effect of 4:1 glyceryl trioleate-glyceryl trierucate (Lorenzo's oil) on disease progression. Eighty-nine boys (mean +/- SD baseline age, 4.7 +/- 4.1 years; range, 0.2-15 years) were identified by a plasma very long-chain fatty acids assay used to screen at-risk boys. All were treated with Lorenzo's oil and moderate fat restriction. Plasma fatty acids and clinical status were followed for 6.9 +/- 2.7 years. Changes in plasma hexacosanoic acid levels were assessed by measuring the length-adjusted area under the curve, and a proportional hazards model was used to evaluate association with the development of abnormal MRI results and neurological abnormalities. Of the 89 boys, 24% developed MRI abnormalities and 11% developed both neurological and MRI abnormalities. Abnormalities occurred only in the 64 patients who were aged 7 years or younger at the time therapy was started. There was significant association between the development of MRI abnormalities and a plasma hexacosanoic acid increase. (For a 0.1-ug/mL increase in the length-adjusted area under the curve for the hexacosanoic acid level, the hazard ratio for incident MRI abnormalities in the whole group was 1.36; P = .01; 95% confidence interval, 1.07-1.72.) Results for patients aged 7 years or younger were similar (P = .04). In this single-arm study, hexacosanoic acid reduction by Lorenzo's oil was associated with reduced risk of developing MRI abnormalities. We recommend Lorenzo's oil therapy in asymptomatic boys with X-linked adrenoleukodystophy who have normal brain MRI results.
Moser HW et al; Arch Neurol 62:1073-80 (2005)
X-linked adrenoleukodystrophy (X-ALD) is an inherited disorder of peroxisomal metabolism, biochemically characterized by deficient beta-oxidation of saturated very long chain fatty acids (VLCFA). The consequent accumulation of these fatty acids in different tissues and in biological fluids is associated with a progressive central and peripheral demyelination, as well as with adrenocortical insufficiency and hypogonadism. Seven variants of this disease have been described, cerebral childhood being the most frequent. The recommended therapy consists of the use of the glyceroltrioleate/glyceroltrierucate mixture known as Lorenzo's Oil (LO), combined with a VLCFA-poor diet, but only in asymptomatic patients will this treatment prevent the progression of the symptomatology. In the present study we evaluated the biochemical course of patients with cerebral childhood (CCER) and asymptomatic clinical forms of X-ALD treated with LO associated with a VLCFA-restricted diet. We observed that hexacosanoic acid plasma concentrations and hexacosanoic/docosanoic ratio were significantly reduced in CCER patients during treatment when compared with diagnosis. Hexacosanoic acid plasma level was significantly reduced when compared with that at diagnosis and achieved the normal levels only in asymptomatic patients under LO treatment. In asymptomatic patients the magnitude of hexacosanoic acid decrease was higher than that of the CCER patients. These results show the good biochemical response of LO treatment in asymptomatic X-ALD patients. It is possible to suppose that this could be correlated with the prevention of the appearance of neurological signals in this group of patients treated with LO.
PMID:18026827 Deon M et al; Metab Brain Dis 23 (1): 43-9 (2008)
/The study/ investigated the possible therapeutic effect of decreasing plasma levels of very-long-chain fatty acids (C26:0) with a synthetic oil containing trioleate and trielucate (Lorenzo's oil) as well as increasing docosahexaenoic acid (DHA) in red blood cells (RBC) with DHA ethyl ester in four patients with Zellweger syndrome. /The study/ investigated serial changes of plasma C26:0 levels and DHA levels in RBC membranes by gas-liquid chromatography/mass spectrometry (GC/MS). After death, the fatty acid composition of each patient's cerebrum and liver was studied. Dietary administration of Lorenzo's oil diminished plasma C26:0 levels. Earlier administration of Lorenzo's oil was more effective and the response did not depend on the duration of administration. DHA was incorporated into RBC membrane lipids when administrated orally, and its level increased for several months. The final DHA level was correlated with the duration of administration and was not related to the timing of initiation of treatment. DHA levels in the brains and livers of treated patients were higher than in untreated patients. Early initiation of Lorenzo's oil and the long-term administration of DHA may be useful for patients with Zellweger syndrome.
Arai Y et al; Congenital Anomalies 48 (4): 180-182 (2008)
In the small intestine, most triglycerides are split into monoglycerides, free fatty acids, and glycerol, which are absorbed by the intestinal mucosa. Within the epithelial cells, resynthesized triglycerides collect into globules along with cholesterol and phospholipids and are encased in a protein coat as chylomicrons. Chylomicrons are transported in the lymph to the thoracic duct and eventually to the venous system. The chylomicrons are removed from the blood as they pass through the capillaries of adipose tissue. Fat is stored in adipose cells until it is transported to other tissues as free fatty acids which are used for cellular energy or incorporated into cell membranes.
Cosmetic Ingredient Review Expert Panel; International Journal of Toxicology: 20 (suppl. 4): 61-94 (2001)
When (14)C-labeled long-chain triglycerides are administered intravenously, 25% to 30% of the radiolabel is found in the liver within 30 to 60 minutes, with less than 5% remaining after 24 hours. Lesser amounts of radiolabel are found in the spleen and lungs. After 24 hours, nearly 50% of the radiolabel has been expired in carbon dioxide, with 1% of the carbon label remaining in the brown fat. The concentration of radioactivity in the epididymal fat is less than half that of the brown fat.
Cosmetic Ingredient Review Expert Panel; International Journal of Toxicology: 20 (suppl. 4): 61-94 (2001)
Rats were fed an emulsion diet (via stomach tube) consisting of 95 parts triolein (Glycerol Trioleate) and 5 parts glycerol 1- (14)C-trioleate. The percentage of administered glycerol 1- (14)C-trioleate that was identified in the lymph in 24 hours was 88%. In an earlier study four male rats (weights 250 g) were dosed orally with [1-(14)C]triolein. The percentage of radioactivity that was absorbed in 24 hours ranged from 57% to 92% (mean =78.2%). The percentage of absorbed activity that was recovered in the lymph fat from the thoracic duct ranged from 51% to 83% (mean =65.5%).
Cosmetic Ingredient Review Expert Panel; International Journal of Toxicology: 20 (suppl. 4): 61-94 (2001)
After a single dose of [1- (14)C]triolein was administered intravenously into fasted rats, a high rate of uptake was noted within the first hour in the following organs: liver, myocardium, gastric mucosa, and diaphragm. However, after 24 hours, radioactivity in these tissues had decreased markedly. A similar pattern of distribution was noted in mice; however, large amounts of radioactivity were also noted in the brown fat, white adipose tissue, and spleen, even after 24 hours.
Cosmetic Ingredient Review Expert Panel; International Journal of Toxicology: 20 (suppl. 4): 61-94 (2001)
For more Absorption, Distribution and Excretion (Complete) data for TRIOLEIN (7 total), please visit the HSDB record page.
Hydrolysis of /Triolein/ by hepatic triacylglycerol lipase in plasma from ICR mice has been demonstrated in vitro.
Cosmetic Ingredient Review Expert Panel; International Journal of Toxicology: 20 (suppl. 4): 61-94 (2001)
The metabolism of triolein in vitro was evaluated using isolated perfusion of a rat liver in tandem with an isolated rat hind-end. This permitted the study of lipid transfer between the two. In the absence of added triolein, a net removal of free fatty acids was demonstrated in both tissue beds when fatty acid gradients across tissue beds were measured. Following the addition of 100 mg of triolein (as [(3)H]-glycerol-[(14)C]triolein) to either reservoir in the system, an appreciable net production of free fatty acid was noted for the hind-end gradient at 30 minutes. This hind-end free fatty acid efflux amounted to more than one third of the catabolism of triolein.
Cosmetic Ingredient Review Expert Panel; International Journal of Toxicology: 20 (suppl. 4): 61-94 (2001)
In experimental studies, embolization of the cerebral hemisphere with triolein emulsion has revealed reversible magnetic resonance imaging (MRI) findings in the subacute stage. /The aim of this study was/ to investigate the changes in the major metabolites, by proton magnetic resonance spectroscopy (MRS), in a cerebral fat embolism induced by a triolein emulsion.The internal carotid arteries of 19 cats were injected with a triolein emulsion, and multivoxel MRS was performed 30 min, 1 day, and 7 days later. In the control group, six cats were injected with normal saline. The MR spectra were evaluated for N-acetyl aspartate (NAA), creatine (Cr), and choline (Cho), along with the presence of lipid and lactate. Semiquantitative analyses of NAA/Cr, Cho/Cr, NAA/Cho, and lipid/Cr ratios compared the median values of the ipsilateral metabolite ratios with those of the contralateral side and in the control group for each point in time.The NAA/Cr, Cho/Cr, and NAA/Cho ratios in the ipsilateral cerebral hemisphere of the embolized group after 30 min, 1 day, and 7days were not significantly different from the contralateral hemisphere of the embolized and control groups (P>0.05). The lipid/Cr ratio in the ipsilateral cerebral hemisphere of the embolized group was significantly higher when compared with the control group (P=0.012 at 30 min, P=0.001 on day 1, and P=0.018 on day 7). Cerebral fat embolism induced by a triolein emulsion resulted in no significant change in the major metabolites of the brain in the acute stage, except for an elevated lipid/Cr ratio, which suggests the absence of any significant hypoxic-ischemic changes in the lesions embolized using a fat emulsion.
PMID:19031181 Baik SK, et al; Acta Radiol 49 (10): 1174-81 (2008)
Effects of protopanaxdiol (PDG) and protopanaxatriol (PTG) types of ginsenosides isolated from the leaves of American ginseng on porcine pancreatic lipase activity were determined in vitro. PDG inhibited the pancreatic lipase activity in a dose-dependent manner at the concentrations of 0.25-1 mg/mL. It inhibited hydrolysis of about 83.2% of triolein at about 1 mg/mL of PDG. However, PTG showed no inhibitory activity. Therefore, anti-obesity activity of PDG was evaluated in mice fed a high-fat diet. The results demonstrated that PDG was effective in preventing and healing obesity, fatty liver and hypertriglyceridemia in mice fed with a high-fat diet.
PMID:20627120 Liu R et al; Fitoterapia 81 (8): 1079-87 (2010)
/Half-life/ 4.5 minutes.
Cosmetic Ingredient Review Expert Panel; International Journal of Toxicology: 20 (suppl. 4): 61-94 (2001)
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
61
PharmaCompass offers a list of Triolein API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Triolein manufacturer or Triolein supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Triolein manufacturer or Triolein supplier.
PharmaCompass also assists you with knowing the Triolein API Price utilized in the formulation of products. Triolein API Price is not always fixed or binding as the Triolein Price is obtained through a variety of data sources. The Triolein Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Triolein manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Triolein, including repackagers and relabelers. The FDA regulates Triolein manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Triolein API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Triolein manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Triolein supplier is an individual or a company that provides Triolein active pharmaceutical ingredient (API) or Triolein finished formulations upon request. The Triolein suppliers may include Triolein API manufacturers, exporters, distributors and traders.
click here to find a list of Triolein suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Triolein DMF (Drug Master File) is a document detailing the whole manufacturing process of Triolein active pharmaceutical ingredient (API) in detail. Different forms of Triolein DMFs exist exist since differing nations have different regulations, such as Triolein USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Triolein DMF submitted to regulatory agencies in the US is known as a USDMF. Triolein USDMF includes data on Triolein's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Triolein USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Triolein suppliers with USDMF on PharmaCompass.
Triolein Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Triolein GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Triolein GMP manufacturer or Triolein GMP API supplier for your needs.
A Triolein CoA (Certificate of Analysis) is a formal document that attests to Triolein's compliance with Triolein specifications and serves as a tool for batch-level quality control.
Triolein CoA mostly includes findings from lab analyses of a specific batch. For each Triolein CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Triolein may be tested according to a variety of international standards, such as European Pharmacopoeia (Triolein EP), Triolein JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Triolein USP).
