
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
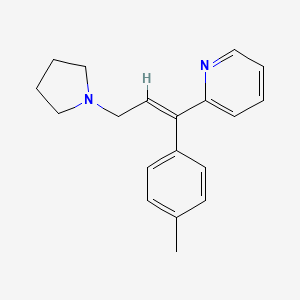

1. Actidil
2. Anhydrous, Triprolidine Hydrochloride
3. Hydrochloride Anhydrous, Triprolidine
4. Hydrochloride, Triprolidine
5. Pro Actidil
6. Triprolidine Hydrochloride
7. Triprolidine Hydrochloride Anhydrous
8. Triprolidine Monohydrochloride
9. Triprolidine Monohydrochloride, (z)-isomer
10. Triprolidine Monohydrochloride, Monohydrate
11. Triprolidine Oxalate
12. Triprolidine Oxalate, (trans)-isomer
13. Triprolidine, (z)-isomer
1. 486-12-4
2. Actidil
3. Triprolidin
4. Triprolidinum
5. Tripolidina
6. Myidyl
7. Histafed
8. Actifed
9. Trans-1-(2-pyridyl)-3-pyrrolidino-1-p-tolylprop-1-ene
10. Actahist
11. Allerfed
12. Corphed
13. Myfed
14. Trilitron
15. Triphed
16. Triprolidine (inn)
17. Trans-1-(4-methylphenyl)-1-(2-pyridyl)-3-pyrrolidinoprop-1-ene
18. 2-[(e)-1-(4-methylphenyl)-3-pyrrolidin-1-ylprop-1-enyl]pyridine
19. Trans-2-(3-(1-pyrrolidinyl)-1-p-tolylpropenyl)pyridine
20. Chembl855
21. (e)-2-(3-(pyrrolidin-1-yl)-1-(p-tolyl)prop-1-en-1-yl)pyridine
22. 2l8t9s52qm
23. Chebi:84116
24. Ncgc00024714-03
25. Nci-c61450
26. Dsstox_cid_3718
27. Triprolidine [inn]
28. Dsstox_rid_77161
29. Dsstox_gsid_23718
30. Triprolidine [inn:ban]
31. Tripolidina [inn-spanish]
32. Triprolidinum [inn-latin]
33. (e)-2-[3-(1-pyrrolidinyl)-1-p-toluenepropenyl]pyridine
34. Hsdb 6316
35. 2-[(1e)-1-(4-methylphenyl)-3-(pyrrolidin-1-yl)prop-1-en-1-yl]pyridine
36. Cas-486-12-4
37. Pyridine, 2-(3-(1-pyrrolidinyl)-1-p-tolylpropenyl)-, (e)-
38. Ccris 7212
39. Einecs 207-627-0
40. Unii-2l8t9s52qm
41. (e)-2-(1-(4-methylphenyl)-3-(1-pyrrolidinyl)-1-propenyl)pyridine
42. 550-70-9
43. Prestwick2_000262
44. Prestwick3_000262
45. Spectrum5_001467
46. Triprolidine [mi]
47. Triprolidine [hsdb]
48. Schembl4905
49. Triprolidine [vandf]
50. Lopac0_001130
51. Bspbio_000104
52. Bspbio_002255
53. (e)-2-(3-(pyrrolidin-1-yl)-1-p-tolylprop-1-enyl)pyridine
54. Bidd:gt0569
55. Triprolidine [who-dd]
56. Bpbio1_000116
57. Gtpl1228
58. Dtxsid3023718
59. Hms2089m21
60. 10191-42-1
61. Hy-b1808
62. Tox21_110919
63. Bdbm50292411
64. Ccg-12422
65. Zinc12503099
66. Tox21_110919_1
67. Db00427
68. Sdccgsbi-0025383.p005
69. Idi1_000297
70. Ncgc00024714-02
71. Ncgc00024714-04
72. Ncgc00024714-05
73. Ncgc00024714-06
74. Ncgc00024714-07
75. Ncgc00024714-08
76. Ncgc00024714-11
77. Ncgc00024714-15
78. Bim-0025383.p001
79. Sbi-0025383.p004
80. Cs-0013854
81. D08648
82. Ab00053563-02
83. Ab00053563-13
84. Ab00053563_14
85. 486t124
86. L000901
87. Q417654
88. 2-(3-pyrrolidin-1-yl-1-p-tolyl-propenyl)-pyridine
89. Brd-k11742128-003-05-1
90. Brd-k11742128-003-15-0
91. 1-((e)-3-pyridin-2-yl-3-p-tolyl-allyl)-pyrrolidinium
92. 2-((e)-3-pyrrolidin-1-yl-1-p-tolyl-propenyl)-pyridine
93. 2-(3-(pyrrolidin-1-yl)-1-p-tolylprop-1-enyl)pyridine
94. 2-[(e)-1-(4-methylphenyl)-3-pyrrolidin-1-yl-prop-1-enyl]pyridine
95. (e)-2-1-(4-methylphenyl)-3-(1-pyrrolidinyl)-1-propenyl-pyridine
| Molecular Weight | 278.4 g/mol |
|---|---|
| Molecular Formula | C19H22N2 |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 278.178298710 g/mol |
| Monoisotopic Mass | 278.178298710 g/mol |
| Topological Polar Surface Area | 16.1 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 336 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Allergic Agents; Histamine H1 Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Antihistamines are indicated in the prophylactic and symptomatic treatment of perennial and seasonal allergic rhinitis, vasomotor rhinitis, and allergic conjunctivitis due to inhalant allergens and foods. /Antihistamines; Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 303
Antihistamines are indicated for the symptomatic treatment of pruritus associated with allergic reactions and of mild, uncomplicated allergic skin manifestations of urticaria and angioedema, in dermatographism, and in urticaria associated with transfusions. /Antihistamines; Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 303
Antihistamines are also used in the treatment of pruritus associated with pityriasis rosea. /Antihistamines; NOT included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 303
For more Therapeutic Uses (Complete) data for TRIPROLIDINE (8 total), please visit the HSDB record page.
Use is not recommended in newborn or premature infants because this age group has an increased susceptibility to anticholinergic side effects, such as central nervous system excitation, and an increased tendency toward convulsions. A paradoxical reaction characterized by hyperexcitability may occur in children taking antihistamines. /Antihistamines/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 306
Dizziness, sedation, confusion, and hypotension may be more likely to occur in geriatric patients taking antihistamines. Geriatric patients are especially susceptible to the anticholinergic side effects, such as dryness of mouth and urinary retention (especially in males), of the antihistamines. If these side effects occur and continue or are severe, medication should probably be discontinued. /Antihistamines/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 306
Prolonged use of antihistamines ... may decrease or inhibit salivary flow, thus contributing to the development of caries, periodontal disease, oral candidiasis, and discomfort. /Antihistamines/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 306
Maternal Medication usually Compatible with Breast-Feeding: Triprolidine: Reported Sign or Symptom in Infant or Effect on Lactation: None. /From Table 6/
Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 142 (1994)
For more Drug Warnings (Complete) data for TRIPROLIDINE (10 total), please visit the HSDB record page.
For the symptomatic relief of seasonal or perennial allergic rhinitis or nonallergic rhinitis; allergic conjunctivitis; and mild, uncomplicated allergic skin manifestations of urticaria and angioedema. Also used in combination with other agents for the symptomatic relief of symptoms associated with the common cold.
In allergic reactions an allergen interacts with and cross-links surface IgE antibodies on mast cells and basophils. Once the mast cell-antibody-antigen complex is formed, a complex series of events occurs that eventually leads to cell-degranulation and the release of histamine (and other chemical mediators) from the mast cell or basophil. Once released, histamine can react with local or widespread tissues through histamine receptors. Histamine, acting on H1-receptors, produces pruritis, vasodilatation, hypotension, flushing, headache, tachycardia, and bronchoconstriction. Histamine also increases vascular permeability and potentiates pain. Triprolidine, is a histamine H1 antagonist that competes with histamine for the normal H1-receptor sites on effector cells of the gastrointestinal tract, blood vessels and respiratory tract. It provides effective, temporary relief of sneezing, watery and itchy eyes, and runny nose due to hay fever and other upper respiratory allergies. Triprolidine has anticholinergic and sedative effects.
Anti-Allergic Agents
Agents that are used to treat allergic reactions. Most of these drugs act by preventing the release of inflammatory mediators or inhibiting the actions of released mediators on their target cells. (From AMA Drug Evaluations Annual, 1994, p475) (See all compounds classified as Anti-Allergic Agents.)
Histamine H1 Antagonists
Drugs that selectively bind to but do not activate histamine H1 receptors, thereby blocking the actions of endogenous histamine. Included here are the classical antihistaminics that antagonize or prevent the action of histamine mainly in immediate hypersensitivity. They act in the bronchi, capillaries, and some other smooth muscles, and are used to prevent or allay motion sickness, seasonal rhinitis, and allergic dermatitis and to induce somnolence. The effects of blocking central nervous system H1 receptors are not as well understood. (See all compounds classified as Histamine H1 Antagonists.)
R - Respiratory system
R06 - Antihistamines for systemic use
R06A - Antihistamines for systemic use
R06AX - Other antihistamines for systemic use
R06AX07 - Triprolidine
Absorption
Rapidly absorbed in the intestinal tract.
The H1 antagonists are well absorbed from the GI tract. Following oral administration, peak plasma concn are achieved in 2 to 3 hr and effects usually last 4 to 6 hr; however, some of the drugs are much longer acting ... . /Histamine Antagonists: H1 Antagonists/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 584
... H1 antagonists are eliminated more rapidly by children than by adults and more slowly in those with severe liver disease. /Histamine Antagonists: H1 Antagonists/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 584
MAIN SITE OF METABOLIC TRANSFORMATION IS LIVER. /ANTIHISTAMINES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 607
4 to 6 hours.
Elimination: 3 to 3.3 hours
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 305
Triprolidine binds to the histamine H1 receptor. This blocks the action of endogenous histamine, which subsequently leads to temporary relief of the negative symptoms brought on by histamine.
Antihistamines used in the treatment of allergy act by competing with histamine for H1-receptor sites on effector cells. They thereby prevent, but do not reverse, responses mediated by histamine alone. Antihistamines antagonize, in varying degrees, most of the pharmacological effects of histamine, including urticaria and pruritus. Also, the anticholinergic actions of most antihistamines provide a drying effect on the nasal mucosa. /Antihistamines/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 304
Antihistamines used in the treatment of allergy act by competing with histamine for H1-receptor sites on effector cells. They thereby prevent, but do not reverse, responses mediated by histamine alone. Antihistamines antagonize, in varying degrees, most of the pharmacological effects of histamine, including urticaria and pruritus. Also, the anticholinergic actions of most antihistamines provide a drying effect on the nasal mucosa. /Antihistamines/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 304
H1 antagonists inhibit most responses of smooth muscle to histamine. Antagonism of the constrictor action of histamine on respiratory smooth muscle is easily shown in vivo and in vitro. /Histamine Antagonists: H1 Antagonists/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 582
/SRP:/ The action of histamine results in increased permeability and formation of edema and wheal. H1 antagonists block that action.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 583
For more Mechanism of Action (Complete) data for TRIPROLIDINE (7 total), please visit the HSDB record page.
ABOUT THIS PAGE
35
PharmaCompass offers a list of Triprolidin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Triprolidin manufacturer or Triprolidin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Triprolidin manufacturer or Triprolidin supplier.
PharmaCompass also assists you with knowing the Triprolidin API Price utilized in the formulation of products. Triprolidin API Price is not always fixed or binding as the Triprolidin Price is obtained through a variety of data sources. The Triprolidin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Triprolidin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Triprolidin, including repackagers and relabelers. The FDA regulates Triprolidin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Triprolidin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Triprolidin supplier is an individual or a company that provides Triprolidin active pharmaceutical ingredient (API) or Triprolidin finished formulations upon request. The Triprolidin suppliers may include Triprolidin API manufacturers, exporters, distributors and traders.
Triprolidin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Triprolidin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Triprolidin GMP manufacturer or Triprolidin GMP API supplier for your needs.
A Triprolidin CoA (Certificate of Analysis) is a formal document that attests to Triprolidin's compliance with Triprolidin specifications and serves as a tool for batch-level quality control.
Triprolidin CoA mostly includes findings from lab analyses of a specific batch. For each Triprolidin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Triprolidin may be tested according to a variety of international standards, such as European Pharmacopoeia (Triprolidin EP), Triprolidin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Triprolidin USP).
