



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
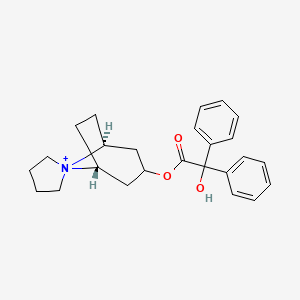

1. Trospium Cation
2. 47608-32-2
3. Trospium Ion
4. T4y8ork057
5. [(1s,5r)-spiro[8-azoniabicyclo[3.2.1]octane-8,1'-azolidin-1-ium]-3-yl] 2-hydroxy-2,2-diphenylacetate
6. Ncgc00167438-01
7. Ncgc00167438-02
8. Unii-t4y8ork057
9. Tropsium Cation
10. Tropsium Ion
11. Trospium [vandf]
12. Trospium [who-dd]
13. Schembl119810
14. Schembl2447242
15. Chembl1888176
16. Chebi:145791
17. Zinc12503068
18. 3alpha-hydroxyspiro(1alphah,5alphah-nortropane-8,1'-pyrrolidinium) Benzilate
19. Zinc100016084
20. Zinc100371992
21. Am84314
22. Db00209
23. Ab01274814-01
24. Ab01274814_02
25. 3.alpha.-hydroxyspiro(1.alpha.h,5.alpha.h-nortropane-8,1'-pyrrolidinium) Benzilate
26. (1alpha,3beta,5alpha)-3-[(2-hydroxy-2,2-diphenylacetyl)oxy]-spiro[8-azoniabicyclo[3.2.1]octane-8,1'-pyrrolidinium]
27. (1alpha,5alpha)-3beta-[(hydroxydiphenylacetyl)oxy]spiro[8-azoniabicyclo[3.2.1]octane-8,1'-pyrrolidinium]
28. (1s,3r,5r)-3-[(2-hydroxy-2,2-diphenylacetyl)oxy]-8lambda(5)-azaspiro[bicyclo[3.2.1]octane-8,1'-pyrrolidin]-8-ylium
29. Spiro(8-azoniabicyclo(3.2.1)octane-8,1'-pyrrolidinium), 3-((hydroxydiphenylacetyl)oxy)-, (1.alpha.,3.beta.,5.alpha.)-
| Molecular Weight | 392.5 g/mol |
|---|---|
| Molecular Formula | C25H30NO3+ |
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 392.22256882 g/mol |
| Monoisotopic Mass | 392.22256882 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 1 |
| Complexity | 553 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency, detrusor instability and frequency of micturition.
FDA Label
Trospium is an antispasmodic, antimuscarinic agent indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency. According to receptor assays, it displays higher affinity towards muscarininc receptors compared to nicotinic receptors at therapeutic concentrations.
G04BD09
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G04 - Urologicals
G04B - Urologicals
G04BD - Drugs for urinary frequency and incontinence
G04BD09 - Trospium
Absorption
9.6%
Route of Elimination
After administration of oral 14C-trospium chloride, the majority of the dose (85.2%) was recovered in feces and a smaller amount (5.8% of the dose) was recovered in urine; 60% of the radioactivity excreted in urine was unchanged trospium. Trospium mainly undergoes elimination via active tubular secretion, as indicated by the mean renal clearance of 29.07 L/hour, which is about 4-fold higher than average glomerular filtration rate. Trospium is metabolized by ester hydrolysis and excreted by the kidneys by a combination of tubular secretion and glomerular filtration.
Volume of Distribution
395 140 L
Clearance
Renal cl=29.07 L/hour
Not fully defined
20 hours
Trospium antagonizes the effect of acetylcholine on muscarinic receptors in cholinergically innervated organs. Its parasympatholytic action reduces the tonus of smooth muscle in the bladder.


