
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
FDA Orange Book

0
Europe

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
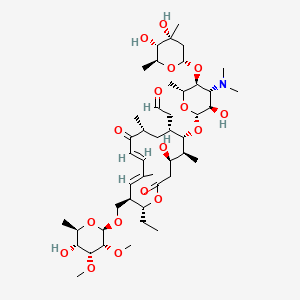

1. Fradizine
2. Hydrochloride, Tylosin
3. Tartrate, Tylosin
4. Tylan
5. Tylosin Hydrochloride
6. Tylosin Tartrate
7. Tylosin Tartrate (salt)
8. Tylosine
1. Tylosin A
2. Tylan
3. Tylosine
4. Tylocine
5. Tylosin Tartrate
6. 1401-69-0
7. Tilosina
8. Tylosinum
9. Fradizine
10. Yef4jxn031
11. Chebi:17658
12. 2-[(4r,5s,6s,7r,9r,11e,13e,15r,16r)-6-[(2r,3r,4r,5s,6r)-5-[(2s,4r,5s,6s)-4,5-dihydroxy-4,6-dimethyloxan-2-yl]oxy-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-16-ethyl-4-hydroxy-15-[[(2r,3r,4r,5r,6r)-5-hydroxy-3,4-dimethoxy-6-methyloxan-2-yl]oxymethyl]-5,9,13-trimethyl-2,10-dioxo-1-oxacyclohexadeca-11,13-dien-7-yl]acetaldehyde
13. 8026-48-0
14. Vubityl 200
15. Nsc-758961
16. Sr-05000002057
17. Tylosine [inn-french]
18. Tylosinum [inn-latin]
19. Tilosina [inn-spanish]
20. Unii-yef4jxn031
21. Tylosin [usp:inn:ban]
22. Hsdb 7022
23. Einecs 215-754-8
24. Tylan (tn)
25. Tylosin A; Fradizine
26. Tylosin (usp/inn)
27. Ai3-29799
28. Tylosin [inn]
29. Tylosin [mi]
30. Tylosin [mart.]
31. Tylosin [usp-rs]
32. Schembl3081
33. Bspbio_003548
34. Tylosin [green Book]
35. Chembl42743
36. Spectrum1505312
37. Tylosin [usp Impurity]
38. Tylosin [usp Monograph]
39. Dtxsid3043996
40. Schembl16931534
41. Hy-b0519a
42. Hms1922b22
43. Hms2093b11
44. Pharmakon1600-01505312
45. Ccg-38343
46. Lmpk04000004
47. Mfcd09881413
48. Nsc758961
49. S5108
50. Akos037515746
51. Zinc238809556
52. Zinc252441679
53. Cs-3442
54. Db11475
55. Ncgc00263955-02
56. (10e,12e)-(3r,4s,5s,6r,8r,14s,15r)-14-((6-deoxy-2,3-di-o-methyl-beta-d-allopyranosyl)oxymethyl)-5-((3,6-dideoxy-4-o-(2,6-dideoxy-3-c-methyl-alpha-l-ribo-hexopyranosyl)-3-dimethylamino-beta-d-glucopyranosyl)oxy)-6-formylmethyl-3-hydroxy-4,8,12-trimet
57. E713
58. Tylosin A 100 Microg/ml In Acetonitrile
59. Sbi-0206754.p001
60. C01457
61. D02490
62. Ab00959655_03
63. Ab00959655_04
64. Q411462
65. Sr-05000002057-1
66. Sr-05000002057-2
67. Sr-05000002057-3
68. Brd-k37753391-046-03-2
69. (10e,12e)-(3r,4s,5s,6r,8r,14s,15r)-14-((6-deoxy-2,3-di-o-methyl-beta-d-allopyranosyl)oxymethyl)-5-((3,6-dideoxy-4-o-(2,6-dideoxy-3-c-methyl-alpha-l-ribo-hexopyranosyl)-3-dimethylamino-beta-d-glucopyranosyl)oxy)-6-formylmethyl-3-hydroxy-4,8,12-trimethyl-9-oxoheptadeca-10,12-dien-15-olide
70. [(2r,3r,4e,6e,9r,11r,12s,13s,14r)-12-[3,6-dideoxy-4-o-(2,6-dideoxy-3-c-methyl-alpha-l-ribo-hexopyranosyl)-3-(dimethylamino)-beta-d-glucopyranosyloxy]-2-ethyl-14-hydroxy-5,9,13-trimethyl-8,16-dioxo-11-(2-oxoethyl)oxacyclohexadeca-4,6-dien-3-yl]methyl 6-deoxy-2,3-di-o-methyl-beta-d-allopyranoside
71. [(2r,3r,4e,6e,9r,11r,12s,13s,14r)-12-{[3,6-dideoxy-4-o-(2,6-dideoxy-3-c-methyl-alpha-l-ribo-hexopyranosyl)-3-(dimethylamino)-beta-d-glucopyranosyl]oxy}-2-ethyl-14-hydroxy-5,9,13-trimethyl-8,16-dioxo-11-(2-oxoethyl)oxacyclohexadeca-4,6-dien-3-yl]methyl 6-deoxy-2,3-di-o-methyl-beta-d-allopyranoside
72. 2-[(4r,5s,6s,7r,9r,11e,13z,15r,16r)-6-[(2r,3r,4r,5s,6r)-5-[(2s,4r,5s,6s)-4,5-dihydroxy-4,6-dimethyloxan-2-yl]oxy-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-16-ethyl-4-hydroxy-15-[[(2r,3r,4r,5r,6r)-5-hydroxy-3,4-dimethoxy-6-methyloxan-2-yl]oxymethyl]-5,9,13-trimethyl-2,10-dioxo-1-oxacyclohexadeca-11,13-dien-7-yl]acetaldehyde
73. 6r)-5-hydroxy-3,4-dimethoxy-6-methyloxan-2-yl]oxymethyl]-5,9,13-trimethyl-2,10-dioxo-1-oxacyclohexadeca-11,13-dien-7-yl]acetaldehyde
74. Antibiotic Obtained From Cultures Of Streptomyces Fradiae, Or The Same Substance Produced By Any Other Means
| Molecular Weight | 916.1 g/mol |
|---|---|
| Molecular Formula | C46H77NO17 |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 18 |
| Rotatable Bond Count | 13 |
| Exact Mass | 915.51914999 g/mol |
| Monoisotopic Mass | 915.51914999 g/mol |
| Topological Polar Surface Area | 239 Ų |
| Heavy Atom Count | 64 |
| Formal Charge | 0 |
| Complexity | 1560 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 21 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Bacterial Agents
National Library of Medicine's Medical Subject Headings. Tylosin. Online file (MeSH, 2015). Available from, as of August 19, 2015: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
MEDICATION (VET): Chickens: As an aid in the treatment of chronic respiratory disease (CRD) associated with Mycoplasma gallisepticum sensitive to tylosin in broiler and replacement chickens. For the control of CRD associated with Mycoplasma gallisepticum sensitive to tylosin at the time of vaccination or other stress in chickens. For the control of CRD associated with Mycoplasma synoviae sensitive to tylosin in broiler chickens. /Included in US product label/
NIH; DailyMed. Current Medication Information for Tylosin Tartrate (Tylosin Tartrate) Powder, for Solution (Updated: May 2015). Available from, as of August 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3035e368-583a-41f5-aa8e-65ba9b2f6a8a
MEDICATION (VET): Turkeys: For the reduction in severity of effects of infectious sinusitis associated with Mycoplasma gallisepticum. /Included in US product label/
NIH; DailyMed. Current Medication Information for Tylosin Tartrate (Tylosin Tartrate) Powder, for Solution (Updated: May 2015). Available from, as of August 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3035e368-583a-41f5-aa8e-65ba9b2f6a8a
MEDICATION (VET): Honey Bees: For the control of American Foulbrood (Paenibacillus larvae). /Included in US product label/
NIH; DailyMed. Current Medication Information for Tylosin Tartrate (Tylosin Tartrate) Powder, for Solution (Updated: May 2015). Available from, as of August 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3035e368-583a-41f5-aa8e-65ba9b2f6a8a
For more Therapeutic Uses (Complete) data for TYLOSIN (6 total), please visit the HSDB record page.
Do not administer orally to rodents or rabbits. Do not administer to horses. Avoid intravenous administration. Do not inject more than 10 mL in one intramuscular site.
Papich, M.G. Saunders Handbook of Veterinary Drugs Small and Large Animal. 3rd ed. St. Louis, MO: Elsevier Saunders, 2011, p. 792
Tylosin is contraindicated in patients hypersensitive to it or other macrolide antibiotics (eg, erythromycin). Most clinicians feel that tylosin is contraindicated in horses, as severe and sometimes fatal diarrheas may result from it use in that species.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 1460
Tylosin may cause diarrhea in some animals. However, oral treatment for colitis in dogs has been administered for several months with safety. Skin reactions have been observed in pigs. Administration to horses has been fatal.
Papich, M.G. Saunders Handbook of Veterinary Drugs Small and Large Animal. 3rd ed. St. Louis, MO: Elsevier Saunders, 2011, p. 792
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
The pharmacokinetics and oral bioavailability of tylosin tartrate and tylosin phosphate were carried out in broiler chickens according to a principle of single dose, random, parallel design. The two formulations of tylosin were given orally and intravenously at a dose level of 10 mg/kg b.w to chicken after an overnight fasting (n = 10 chickens/group). Serial blood samples were collected at different time points up to 24 hr postdrug administration. A high performance liquid chromatography method was used for the determination of tylosin concentrations in chicken plasma. The tylosin plasma concentration's time plot of each chicken was analyzed by the 3P97 software. The pharmacokinetics of tylosin was best described by a one-compartmental open model 1st absorption after oral administration. After intravenous administration the pharmacokinetics of tylosin was best described by a two-compartmental open model, and there were no significant differences between tylosin tartrate and tylosin phosphate. After oral administration, there were significant differences in the Cmax (0.18 + or - 0.01, 0.44 + or - 0.09) and AUC (0.82 + or - 0.05, 1.57 + or - 0.25) between tylosin phosphate and tylosin tartrate. The calculated oral bioavailability (F) of tylosin tartrate and tylosin phosphate were 25.78% and 13.73%, respectively. Above all, we can reasonably conclude that, the absorption of tylosin tartrate is better than tylosin phosphate after oral administration.
PMID:24325541 Ji LW et al; J Vet Pharmacol Ther 37 (3): 312-5 (2014)
/MILK/ The aim of this study is to determine the pharmacokinetics of tylosin and tilmicosin in serum and milk in healthy Holstein breed cows (n = 12) and reevaluate the amount of residue in milk. Following the intramuscular administration of tylosin, the maximum concentrations (C max) in serum and milk were found to be 1.30 + or - 0.24 and 4.55 + or - 0.23 ug/mL, the time required to reach the peak concentration (t max) was found to be 2nd and 4th hour, and elimination half-live were found to be 20.46 + or - 2.08 and 26.36 + or - 5.55 hour, respectively. Following the subcutaneous administration of tilmicosin, the C max in serum and milk were found to be 0.86 + or - 0.20 and 20.16 + or - 1.13 ug/mL, the t max was found to be 1st and 8th hr, and the elimination half life were found to be 29.94 + or - 6.65 and 43.02 + or - 5.18 hr, respectively. AUCmilk/AUCserum and C max-milk/C max-serum rates, which are indicators for determining the rate of drugs that pass into milk, were, respectively, calculated as 5.01 + or - 0.72 and 3.61 + or - 0.69 for tylosin and 23.91 + or - 6.38 and 20.16 + or - 1.13 for tilmicosin. In conclusion, it may be stated that milk concentration of tylosin after parenteral administration is higher than expected like tilmicosin and needs more withdrawal period for milk than reported.
PMID:25177733 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4142165 Avci T, Elmas M; ScientificWorldJournal. 2014;2014:869096. doi: 10.1155/2014/869096. Epub 2014
Biological availability and pharmacokinetic properties of tylosin were determined in broiler chickens after oral and iv administration at a dose of 10 mg/kg. The calculated bioavailability--F%, by comparing AUC values--oral and AUC--iv, ranged from 30%-34%. After intravenous injection tylosin was rapidly distributed in the organism, showing elimination half-life values of 0.52 hr and distribution volume (Vd) of 0.69 L/kg, at a clearance rate (Cl) of 5.30 +/- 0.59 mL/min/kg. After oral administration, tylosin has a similar distribution volume (Vd = 0.85 L/kg), while the elimination half-life of 2.07 hr was four times bigger than after iv administration at Cl = 4.40 +/- 0.27 mL/min/kg. The obtained value tmax = 1.5 hr for tylosin after oral administration indicates that using this antibiotic with drinking water in broiler chickens is the method of choice. However, a relatively low value Cmax = 1.2 micrograms/ml after oral administration of tylosin shows that dosing of this antibiotic in broiler chickens should be higher than in other food producing animals.
PMID:12448074 Kowalski C et al; Pol J Vet Sci 5 (3): 127-30 (2002)
/MILK/ Antibiotic residues in milk above tolerance levels interfere with dairy product processing and pose potential health risks to consumers. Residue avoidance programmes include, among other components, the observance of withdrawal times indicated in label instructions. Persistence of antibiotics in milk following treatment is influenced by drug, dosage, route of administration, body weight and mammary gland health status. Compositional changes that take place during intramammary infection (IMI) can affect antibiotic excretion in milk, thus modifying milk withdrawal time. The objectives of this study were to validate sensitivity and specificity of a qualitative microbiological method (Charm AIM-96) to detect tylosin in bovine composite milk and to determine the influence of subclinical IMI in tylosin excretion following intramuscular administration. For test validation, two groups of approximately 120 cows were used; one received a single intramuscular injection of tylosin tartrate at a dose of 20 mg/kg, while the other group remained as untreated control. Test sensitivity and specificity were 100% and 94.1% respectively. To determine the influence of subclinical IMI in tylosin excretion, two groups of seven cows, one with somatic cell counts (SCC) < or =250 000 cells/ml and the other with SCC > or =900 000, were administered a single intramuscular injection of tylosin tartrate at a dose of 20 mg/kg. Milk samples were obtained every 12 h for 10 days following treatment. Milk tylosin excretion averaged between 5 and 9 days for cows with low and high SCC respectively (P < 0.0001). Compositional changes in cows with high SCC most likely affect the pharmacokinetic characteristics of tylosin, extending the presence of the antibiotic in milk, thus influencing milk withdrawal times.
PMID:17359452 Litterio NJ et al; J Vet Med A Physiol Pathol Clin Med 54 (1): 30-5 (2007)
For more Absorption, Distribution and Excretion (Complete) data for TYLOSIN (10 total), please visit the HSDB record page.
The tylosin-biosynthetic (tyl) gene cluster of Streptomyces fradiae contains ancillary genes that encode functions normally associated with primary metabolism. These can be disrupted without loss of viability, since equivalent genes (presumably used for 'housekeeping' purposes) are also present elsewhere in the genome. The tyl cluster also contains two genes that encode products unlike any proteins in the databases. Two ancillary genes, metF (encoding N5,N10-methylenetetrahydrofolate reductase) and metK, encoding S-adenosylmethionine synthase, flank one of the 'unknown' genes (orf9) in the tyl cluster. In a strain of S. fradiae in which all three of these genes were disrupted, tylosin production was reduced, although this effect was obscured in media supplemented with glycine betaine which can donate methyl groups to the tetrahydrofolate pool. Apparently, one consequence of the recruitment of ancillary genes into the tyl cluster is enhanced capacity for transmethylation during secondary metabolism.
PMID:11594346 Butler AR et al; J Antibiot (Tokyo) 54 (8): 642-9 (2001)
Studies on the susceptibility of pathogenic Nocardia to macrolide antibiotics, chalcomycin and tylosin, showed that most of the Nocardia species examined were highly resistant to both antibiotics, although N. nova was moderately susceptible. N. asteroides IFM 0339 converted these macrolides into inactive metabolites by glycosylation at 2'-OH or glycosylation and reduction of the 20-formyl group. The structures of the metabolites were determined from NMR and MS data to be 2'-[O-(beta-D-glucopyranosyl)]chalcomycin (2), 2'-[O-(beta-D-glucopyranosyl)]tylosin (5) and 20-dihydro-2'-[O-(beta-D-glucopyranosyl)]tylosin (4).
PMID:11302489 Morisaki N et al; J Antibiot (Tokyo) 54 (2): 157-65 (2001)
Tylosin is produced by Streptomyces fradiae via a combination of polyketide metabolism and synthesis of three deoxyhexose sugars, of which mycaminose is the first to be added to the polyketide aglycone, tylactone (protylonolide). Previously, disruption of the gene (tylMII) encoding attachment of mycaminose to the aglycone unexpectedly abolished accumulation of the latter, raising the possibility of a link between polyketide metabolism and deoxyhexose biosynthesis in S. fradiae. However, at that time, it was not possible to eliminate an alternative explanation, namely, that downstream effects on the expression of other genes, not involved in mycaminose metabolism, might have contributed to this phenomenon. Here, it is shown that disruption of any of the four genes (tylMI--III and tylB) specifically involved in mycaminose biosynthesis elicits a similar response, confirming that production of mycaminosyl-tylactone directly influences polyketide metabolism in S. fradiae. Under similar conditions, when mycaminose biosynthesis was specifically blocked by gene disruption, accumulation of tylactone could be restored by exogenous addition of glycosylated tylosin precursors. Moreover, certain other macrolides, not of the tylosin pathway, were also found to elicit qualitatively similar effects. Comparison of the structures of stimulatory macrolides will facilitate studies of the stimulatory mechanism.
PMID:11283275 Butler AR et al; Microbiology 147 (Pt 4): 795-801 (2001)
Three glycosyltransferases are involved in tylosin biosynthesis in Streptomyces fradiae. The first sugar to be added to the polyketide aglycone (tylactone) is mycaminose and the gene encoding mycaminosyltransferase is orf2* (tylM2). However, targeted disruption of orf2* did not lead to the accumulation of tylactone under conditions that normally favor tylosin production; instead, the synthesis of tylactone was virtually abolished. This may, in part, have resulted from a polar effect on the expression of genes downstream of orf2*, particularly orf4* (ccr) which encodes crotonyl-CoA reductase, an enzyme that supplies 4-carbon extender units for polyketide metabolism. However, that cannot be the entire explanation, since tylosin production was restored at about 10% of the wild-type level when orf2* was re-introduced into the disrupted strain. When glycosylated precursors of tylosin were fed to the disrupted strain, they were converted to tylosin, confirming that two of the three glycosyltransferase activities associated with tylosin biosynthesis were still intact. Interestingly, however, tylactone also accumulated under such conditions and, to a much lesser extent, when tylosin was added to similar fermentations. It is concluded that glycosylated macrolides exert a pronounced positive effect on polyketide metabolism in S. fradiae.
PMID:9421911 Fish SA, Cundliffe E; Microbiology 143 (Pt 12): 3871-6 (1997)
For more Metabolism/Metabolites (Complete) data for TYLOSIN (6 total), please visit the HSDB record page.
Biological availability and pharmacokinetic properties of tylosin were determined in broiler chickens after oral and iv administration at a dose of 10 mg/kg. ... After intravenous injection, tylosin ... /had an/ elimination half-life value of 0.52 .. . After oral administration, tylosin /had an / elimination half-life of 2.07 hr ... .
PMID:12448074 Kowalski C et al; Pol J Vet Sci 5 (3): 127-30 (2002)
The elimination half-life of tylosin is reportedly 54 minutes in small animals, 139 minutes in newborn calves, and 64 minutes in calves 2 months of age or older.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 1460
The inhibition of peptide bond formation by tylosin, a 16-membered ring macrolide, was studied in a model system derived from Escherichia coli. In this cell-free system, a peptide bond is formed between puromycin (acceptor substrate) and AcPhe-tRNA (donor substrate) bound at the P-site of poly(U)-programmed ribosomes. It is shown that tylosin inhibits puromycin reaction as a slow-binding, slowly reversible inhibitor. Detailed kinetic analysis reveals that tylosin (I) reacts rapidly with complex C, i.e., the AcPhe-tRNA. poly(U).70S ribosome complex, to form the encounter complex CI, which then undergoes a slow isomerization and is converted to a tight complex, CI, inactive toward puromycin. These events are described by the scheme C + I <==> (K(i)) CI <==> (k(4), k(5)) CI. The K(i), k(4), and k(5) values are equal to 3 microM, 1.5 min(-1), and 2.5 x 10(-3) min(-1), respectively. The extremely low value of k(5) implies that the inactivation of complex C by tylosin is almost irreversible. The irreversibility of the tylosin effect on peptide bond formation is significant for the interpretation of this antibiotic's therapeutic properties; it also renders the tylosin reaction a useful tool in the study of other macrolides failing to inhibit the puromycin reaction but competing with tylosin for common binding sites on the ribosome. Thus, the tylosin reaction, in conjunction with the puromycin reaction, was applied to investigate the erythromycin mode of action. It is shown that erythromycin (Er), like tylosin, interacts with complex C according to the kinetic scheme C + Er <==> (K(er)) CEr <==> (k(6), k(7)) C*Er and forms a tight complex, CEr, which remains active toward puromycin. The determination of K(er), k(6), and k(7) enables us to classify erythromycin as a slow-binding ligand of ribosomes
PMID:10995229 Dinos GP, Kalpaxis DL; Biochemistry 39 (38): 11621-8 (2000)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
42
PharmaCompass offers a list of Tylosin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Tylosin manufacturer or Tylosin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Tylosin manufacturer or Tylosin supplier.
PharmaCompass also assists you with knowing the Tylosin API Price utilized in the formulation of products. Tylosin API Price is not always fixed or binding as the Tylosin Price is obtained through a variety of data sources. The Tylosin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tylosin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tylosin, including repackagers and relabelers. The FDA regulates Tylosin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tylosin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Tylosin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Tylosin supplier is an individual or a company that provides Tylosin active pharmaceutical ingredient (API) or Tylosin finished formulations upon request. The Tylosin suppliers may include Tylosin API manufacturers, exporters, distributors and traders.
click here to find a list of Tylosin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Tylosin DMF (Drug Master File) is a document detailing the whole manufacturing process of Tylosin active pharmaceutical ingredient (API) in detail. Different forms of Tylosin DMFs exist exist since differing nations have different regulations, such as Tylosin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Tylosin DMF submitted to regulatory agencies in the US is known as a USDMF. Tylosin USDMF includes data on Tylosin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Tylosin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Tylosin suppliers with USDMF on PharmaCompass.
A Tylosin CEP of the European Pharmacopoeia monograph is often referred to as a Tylosin Certificate of Suitability (COS). The purpose of a Tylosin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Tylosin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Tylosin to their clients by showing that a Tylosin CEP has been issued for it. The manufacturer submits a Tylosin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Tylosin CEP holder for the record. Additionally, the data presented in the Tylosin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Tylosin DMF.
A Tylosin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Tylosin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Tylosin suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Tylosin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Tylosin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Tylosin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Tylosin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Tylosin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Tylosin suppliers with NDC on PharmaCompass.
Tylosin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tylosin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tylosin GMP manufacturer or Tylosin GMP API supplier for your needs.
A Tylosin CoA (Certificate of Analysis) is a formal document that attests to Tylosin's compliance with Tylosin specifications and serves as a tool for batch-level quality control.
Tylosin CoA mostly includes findings from lab analyses of a specific batch. For each Tylosin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tylosin may be tested according to a variety of international standards, such as European Pharmacopoeia (Tylosin EP), Tylosin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tylosin USP).

