
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
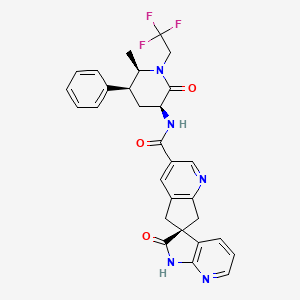

1. 1',2',5,7-tetrahydro-n-((3s,5s,6r)-6-methyl-2-oxo-5-phenyl-1-(2,2,2-trifluoroethyl)-3-piperidinyl)-2'-oxo-, (6s)- Spiro(6h-cyclopenta(b)pyridine-6,3'-(3h)pyrrolo(2,3-b)pyridine)-3-carboxamide
2. Mk-1602
3. Ubrelvy
1. 1374248-77-7
2. Ubrelvy
3. Mk-1602
4. Ubrogepant Anhydrous
5. Ad0o8x2qjr
6. (3's)-n-((3s,5s,6r)-6-methyl-2-oxo-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-3-yl)-2'-oxo-1',2',5,7-tetrahydrospiro(cyclopenta(b)pyridine-6,3'-pyrrolo(2,3-b)pyridine)-3-carboxamide
7. (3s)-n-[(3s,5s,6r)-6-methyl-2-oxo-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-3-yl]-2-oxospiro[1h-pyrrolo[2,3-b]pyridine-3,6'-5,7-dihydrocyclopenta[b]pyridine]-3'-carboxamide
8. Spiro(6h-cyclopenta(b)pyridine-6,3'-(3h)pyrrolo(2,3-b)pyridine)-3-carboxamide, 1',2',5,7-tetrahydro-n-((3s,5s,6r)-6-methyl-2-oxo-5-phenyl-1-(2,2,2-trifluoroethyl)-3-piperidinyl)-2'-oxo-, (3's)-
9. Ubrogepant [usan]
10. Ubrogepant [usan:inn]
11. Unii-ad0o8x2qjr
12. Ubrelvy (tn)
13. Ubrogepant [mi]
14. Ubrogepant [inn]
15. Ubrogepant (usan/inn)
16. Ubrogepant [who-dd]
17. Schembl3698428
18. Chembl2364638
19. Ubrogepant [orange Book]
20. Gtpl10176
21. Dtxsid00160178
22. Ex-a3049
23. Mfcd28386182
24. Mk1602
25. Zinc95598454
26. At16059
27. Db15328
28. Ac-31965
29. Hy-12366
30. Cs-0011109
31. D10673
32. A934103
33. Q27273878
34. S-1374248-77-7
35. (6s)-n-[(3s,5s,6r)-6-methyl-2-oxo-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-3-yl]-2'-oxo-1',2',5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3-b]pyridine]-3-carboxamide
36. (s)-n-((3s,5s,6r)-6-methyl-2-oxo-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-3-yl)-2'-oxo-1',2',5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3-b]pyridine]-3-carboxamide
| Molecular Weight | 549.5 g/mol |
|---|---|
| Molecular Formula | C29H26F3N5O3 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 549.19877419 g/mol |
| Monoisotopic Mass | 549.19877419 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 1000 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ubrogepant is indicated for the acute treatment of migraine with or without aura in adults.
Ubrogepant acutely treats migraine headache pain by blocking the activity of a key transmitter involved in migraine pathogenesis. Exposure to ubrogepant can be significantly increased in patients with severe hepatic or renal insufficiency - dose adjustments are required for these patients in order to avoid excessive exposure, and ubrogepant is not recommended in patients with end-stage renal disease.
N - Nervous system
N02 - Analgesics
N02C - Antimigraine preparations
N02CD - Calcitonin gene-related peptide (cgrp) antagonists
N02CD04 - Ubrogepant
Absorption
Following oral administration, Tmax occurs between 0.7 and 1.5 h. When administered with a high-fat meal, Tmax is delayed by approximately 2 hours and Cmax was reduced by 22% with no significant changes to the AUC. Ubrogepant exhibits dose-proportional pharmacokinetics throughout the entirety of its recommended dosing range.
Route of Elimination
The main route of elimination is fecal/biliary, while renal excretion is comparatively minor - following administration of a single oral dose to healthy subjects, approximately 42% of the dose was recovered unchanged in the feces and 6% was recovered unchanged in the urine.
Volume of Distribution
The apparent central volume of distribution following oral administration is approximately 350 L.
Clearance
The apparent oral clearance of ubrogepant is approximately 87 L/h.
Ubrogepant is eliminated primarily via metabolism, the majority of which is mediated by CYP3A4. Two circulating glucuronide conjugates, along with unchanged parent drug, were found to be the most abundant circulating components in human plasma. The glucuronide metabolites reportedly carry 6000-fold less activity at CGRP receptors and are therefore considered to be pharmacologically inert.
Ubrogepant has an elimination half-life of 5-7 hours.
The currently accepted theory of migraine pathophysiology considers dysfunction of the central nervous system, in particular the trigeminal ganglion, to be the root cause behind the condition. Activation of the trigeminal ganglion triggers the stimulation of trigeminal afferents that project to the spinal cord and synapse on various pain-sensing intra- and extracranial structures, such as the dura mater. Pain signals are then further transmitted via second-order ascending neurons to the brainstem, hypothalamus, and thalamic nuclei, and from there to several cortical regions (e.g. auditory, visual, motor cortices). The trigeminal ganglion appears to amplify and perpetuate the migraine headache pain through the activation of perivascular fibers and the release of molecules involved in pain generation, such as calcitonin gene-related peptide (CGRP). The -isoform of CGRP, expressed in primary sensory neurons, is a potent vasodilator and has been implicated in migraine pathogenesis - CGRP levels are acutely elevated during migraine attacks, return to normal following treatment with triptan medications, and intravenous infusions of CGRP have been shown to trigger migraine-like headaches in migraine patients. In addition to its vasodilatory properties, CGRP appears to be a pronociceptive factor that modulates neuronal excitability to facilitate pain responses. Ubrogepant is a potent antagonist of the calcitonin gene-related peptide receptor - it competes with CGRP for occupancy at these receptors, preventing the actions of CGRP and its ability to amplify and perpetuate migraine headache pain, ultimately terminating the headache.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
63
PharmaCompass offers a list of Ubrogepant API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ubrogepant manufacturer or Ubrogepant supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ubrogepant manufacturer or Ubrogepant supplier.
PharmaCompass also assists you with knowing the Ubrogepant API Price utilized in the formulation of products. Ubrogepant API Price is not always fixed or binding as the Ubrogepant Price is obtained through a variety of data sources. The Ubrogepant Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ubrogepant manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ubrogepant, including repackagers and relabelers. The FDA regulates Ubrogepant manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ubrogepant API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ubrogepant manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ubrogepant supplier is an individual or a company that provides Ubrogepant active pharmaceutical ingredient (API) or Ubrogepant finished formulations upon request. The Ubrogepant suppliers may include Ubrogepant API manufacturers, exporters, distributors and traders.
click here to find a list of Ubrogepant suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ubrogepant DMF (Drug Master File) is a document detailing the whole manufacturing process of Ubrogepant active pharmaceutical ingredient (API) in detail. Different forms of Ubrogepant DMFs exist exist since differing nations have different regulations, such as Ubrogepant USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ubrogepant DMF submitted to regulatory agencies in the US is known as a USDMF. Ubrogepant USDMF includes data on Ubrogepant's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ubrogepant USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ubrogepant suppliers with USDMF on PharmaCompass.
A Ubrogepant written confirmation (Ubrogepant WC) is an official document issued by a regulatory agency to a Ubrogepant manufacturer, verifying that the manufacturing facility of a Ubrogepant active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Ubrogepant APIs or Ubrogepant finished pharmaceutical products to another nation, regulatory agencies frequently require a Ubrogepant WC (written confirmation) as part of the regulatory process.
click here to find a list of Ubrogepant suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ubrogepant as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ubrogepant API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ubrogepant as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ubrogepant and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ubrogepant NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ubrogepant suppliers with NDC on PharmaCompass.
Ubrogepant Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ubrogepant GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ubrogepant GMP manufacturer or Ubrogepant GMP API supplier for your needs.
A Ubrogepant CoA (Certificate of Analysis) is a formal document that attests to Ubrogepant's compliance with Ubrogepant specifications and serves as a tool for batch-level quality control.
Ubrogepant CoA mostly includes findings from lab analyses of a specific batch. For each Ubrogepant CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ubrogepant may be tested according to a variety of international standards, such as European Pharmacopoeia (Ubrogepant EP), Ubrogepant JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ubrogepant USP).

