
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
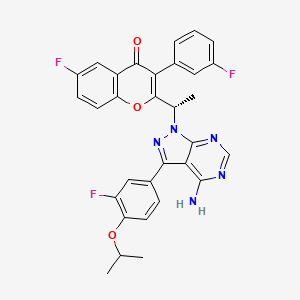

1. Tgr-1202
2. Ukoniq
1. 1532533-67-7
2. Tgr-1202
3. Rp-5264
4. Tgr1202
5. Rp5264
6. Tgr-1202 Free Base
7. Ukoniq
8. Umbralisib [usan]
9. (s)-2-(1-(4-amino-3-(3-fluoro-4-isopropoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-1-yl)ethyl)-6-fluoro-3-(3-fluorophenyl)-4h-chromen-4-one
10. Tgr 1202
11. Tgr-1202 Base
12. 38073mqb2a
13. 2-[(1s)-1-[4-amino-3-(3-fluoro-4-propan-2-yloxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]ethyl]-6-fluoro-3-(3-fluorophenyl)chromen-4-one
14. 2-((1s)-1-(4-amino-3-(3-fluoro-4-(1-methylethoxy)phenyl)-1h-pyrazolo(3,4-d)pyrimidin-1-yl)ethyl)-6-fluoro-3-(3-fluorophenyl)-4h-1-benzopyran-4-one
15. Umbralisib [inn]
16. Umbralisib (usan/inn)
17. Umbralisib [who-dd]
18. Unii-38073mqb2a
19. Gtpl8916
20. Chembl3948730
21. Schembl15557416
22. Tgr 1202 [who-dd]
23. Bdbm184556
24. Dtxsid601337137
25. Bcp24686
26. Ex-a1645
27. Mfcd28386165
28. Nsc793696
29. Nsc800405
30. S8194
31. Example A1 [us2014011819]
32. Zinc141831516
33. Ccg-270101
34. Cs-5243
35. Db14989
36. Nsc-793696
37. Nsc-800405
38. Rp 5264
39. Ac-33183
40. As-52257
41. Hy-12279
42. Us9150579, B1
43. D11322
44. P14656
45. A901657
46. Q27088612
47. (s)-2-(1-(4-amino-3-(3-fluoro-4-isopropoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-1-yl)ethyl)-6-fluoro-3-(3-fluorophenyl)-4h-chromen-4-one;rp-5264
48. 4h-1-benzopyran-4-one, 2-((1s)-1-(4-amino-3-(3-fluoro-4-(1-methylethoxy)phenyl)-1h-pyrazolo(3,4-d)pyrimidin-1-yl)ethyl)-6-fluoro-3-(3-fluorophenyl)-
| Molecular Weight | 571.5 g/mol |
|---|---|
| Molecular Formula | C31H24F3N5O3 |
| XLogP3 | 5.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 6 |
| Exact Mass | 571.18312413 g/mol |
| Monoisotopic Mass | 571.18312413 g/mol |
| Topological Polar Surface Area | 105 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 1020 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Umbralisib is indicated to treat relapsed or refractory marginal zone lymphoma (MZL) in patients who have received at least one prior anti-CD20-based regimen. It is also indicated for the treatment of relapsed or refractory follicular lymphoma (FL) who have received at least three prior lines of systemic therapy.
Umbralisib acts against against marginal zone lymphoma by interrupting the PI3K pathway; this is an essential pathway for B-cell receptor signaling responsible for the progression of lymphoma. In addition, Umbralisib inhibits other pathways involved in specific types of lymphoma, including the casein kinase pathway. An overall response rate of 55% was recorded during clinical trials and the rate of 1-year progression free survival from marginal zone lymphoma was 71%. A relationship between higher umbralisib steady state exposures and higher incidence of adverse reactions, including diarrhea and elevated AST/ALT was observed during clinical studies. The effect of this drug on QT interval has not been fully characterized.
Absorption
Umbralisib is rapidly absorbed in the GI tract. The Tmax of umbralisib is about 4 hours. After consumption of a high-fat, high calorie meal with umbralisib, the AUC increased by 61% and the Cmax increased by 115%.
Route of Elimination
During pharmacokinetic studies, about 81% of the umbralisib dose was recovered in feces (17% unchanged). Approximately 3% was detected in the urine (0.02% unchanged) after a radiolabeled dose of 800 mg in healthy volunteers.
Volume of Distribution
The average apparent central volume of distribution of umbralisib is 312 L.
Clearance
The average apparent clearance of umbralisib is 15.5 L/h.
During in vitro studies, umbralisib was metabolized by CYP2C9, CYP3A4, and CYP1A2 enzymes.
The effective half-life of Umbralisib is about 91 hours.
The PI3K pathway is a deregulated in malignancies, leading to the overexpression of p110 isoforms (p110, p110, p110, p110) that induces malignant transformation in cells. Umbralisib inhibits several protein kinases, including PI3K and casein kinase CK1. PI3K is expressed in both healthy cells and malignant B-cells. CK1 is believed to be involved in the pathogenesis of malignant cells, including lymphomas. This results in reduced progression of relapsed or refractory lymphoma. In biochemical assays, umbralisib inhibited a mutated form of ABL1. In vitro, umbralisib inhibits malignant cell proliferation, CXCL12-mediated cell adhesion, and CCL19-mediated cell migration.
Global Sales Information

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
98
PharmaCompass offers a list of Umbralisib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Umbralisib manufacturer or Umbralisib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Umbralisib manufacturer or Umbralisib supplier.
PharmaCompass also assists you with knowing the Umbralisib API Price utilized in the formulation of products. Umbralisib API Price is not always fixed or binding as the Umbralisib Price is obtained through a variety of data sources. The Umbralisib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Umbralisib manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Umbralisib, including repackagers and relabelers. The FDA regulates Umbralisib manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Umbralisib API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Umbralisib supplier is an individual or a company that provides Umbralisib active pharmaceutical ingredient (API) or Umbralisib finished formulations upon request. The Umbralisib suppliers may include Umbralisib API manufacturers, exporters, distributors and traders.
Umbralisib Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Umbralisib GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Umbralisib GMP manufacturer or Umbralisib GMP API supplier for your needs.
A Umbralisib CoA (Certificate of Analysis) is a formal document that attests to Umbralisib's compliance with Umbralisib specifications and serves as a tool for batch-level quality control.
Umbralisib CoA mostly includes findings from lab analyses of a specific batch. For each Umbralisib CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Umbralisib may be tested according to a variety of international standards, such as European Pharmacopoeia (Umbralisib EP), Umbralisib JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Umbralisib USP).
