
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
Weekly News Recap #Phispers
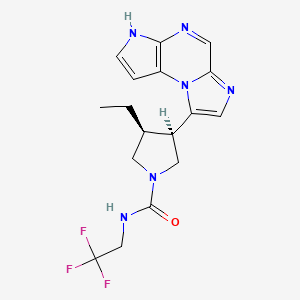

1. (3s,4r)-3-ethyl-4-(3h-imidazo(1,2-a)pyrrolo(2,3-e)pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)-1-pyrrolidinecarboxamide
2. Abt-494
3. Rinvoq
1. 1310726-60-3
2. Abt-494
3. Rinvoq
4. Upadacitinib Anhydrous
5. 4ra0kn46e0
6. (3s,4r)-3-ethyl-4-(1,5,7,10-tetrazatricyclo[7.3.0.02,6]dodeca-2(6),3,7,9,11-pentaen-12-yl)-n-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide
7. (3s,4r)-3-ethyl-4-(3h-imidazo(1,2-a)pyrrolo(2,3-e)pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)-1-pyrrolidinecarboxamide
8. (3s,4r)-3-ethyl-4-(3h-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide
9. 1-pyrrolidinecarboxamide, 3-ethyl-4-(3h-imidazo(1,2-a)pyrrolo(2,3-e)pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)-, (3s,4r)-
10. Mfcd30502663
11. Upadacitinib [usan]
12. Upadacitinib [mi]
13. Upadacitinib, Abt-494
14. Upadacitinib (usan/inn)
15. Upadacitinib [inn]
16. Upadacitinib [usan:inn]
17. Upadacitinib (abt-494)
18. Unii-4ra0kn46e0
19. Upadacitinib [who-dd]
20. Gtpl9246
21. Schembl9991056
22. Chembl3622821
23. Abt 494
24. Dtxsid901027919
25. Upadacitinib [orange Book]
26. Amy16528
27. Bcp19011
28. Ex-a1628
29. Bdbm50503287
30. S8162
31. Cs-6150
32. Db15091
33. Abbv-599 Component Upadacitinib
34. Abt494;abt-494;abt 494
35. Ncgc00588874-01
36. Ac-30326
37. As-56379
38. Hy-19569
39. Upadacitinib Component Of Abbv-599
40. J3.590.729g
41. C72237
42. D10994
43. Q27074125
44. (3s,4r)-3-ethyl-4-(3h-imidazo(1,2-a)pyrrolo(2,3-e)pyrazin- 8-yl)-n-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide Tyrosine Kinase Inhibitor
45. (3s,4r)-3-ethyl-4-(3h-imidazo(1,2-a)pyrrolo(2,3-e)pyrazin-8-yl)-n-(2,2,2- Trifluoroethyl)pyrrolidine-1-carboxamide
46. Rel-(-)-(3s,4r)-3-ethyl-4-(3h-imidazo(1,2-a)pyrrolo(2,3-e)pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide
| Molecular Weight | 380.4 g/mol |
|---|---|
| Molecular Formula | C17H19F3N6O |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 380.15724374 g/mol |
| Monoisotopic Mass | 380.15724374 g/mol |
| Topological Polar Surface Area | 78.3 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 561 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Upadacitinib is indicated for the treatment of moderately to severely active rheumatoid arthritis or active psoriatic arthritis in adult patients who have had an inadequate response or intolerance to one or more disease-modifying anti-rheumatic drugs (DMARDs), such as methotrexate or TNF blockers. For these indications, upadacitinib may be used as monotherapy or in combination with methotrexate. Upadacitinib is also indicated for the treatment of active ankylosing spondylitis in adult patients who have an inadequate response to conventional therapy and for the treatment of moderate to severe atopic dermatitis in patients aged 12 years and older who are candidates for systemic therapy. Combining upadacitinib with other JAK inhibitors, biologic DMARDs, or other potent immunosuppressive agents is not recommended.
FDA Label
Rheumatoid arthritis
RINVOQ is indicated for the treatment of moderate to severe active rheumatoid arthritis in adult patients who have responded inadequately to, or who are intolerant to one or more disease-modifying anti-rheumatic drugs (DMARDs). RINVOQ may be used as monotherapy or in combination with methotrexate.
Psoriatic arthritis
RINVOQ is indicated for the treatment of active psoriatic arthritis in adult patients who have responded inadequately to, or who are intolerant to one or more DMARDs. RINVOQ may be used as monotherapy or in combination with methotrexate.
Axial spondyloarthritis
Non-radiographic axial spondyloarthritis (nr-axSpA)
RINVOQ is indicated for the treatment of active non-radiographic axial spondyloarthritis in adult patients with objective signs of inflammation as indicated by elevated C-reactive protein (CRP) and/or magnetic resonance imaging (MRI), who have responded inadequately to nonsteroidal anti-inflammatory drugs (NSAIDs).
Ankylosing spondylitis (AS, radiographic axial spondyloarthritis )
RINVOQ is indicated for the treatment of active ankylosing spondylitis in adult patients who have responded inadequately to conventional therapy.
Atopic dermatitis
RINVOQ is indicated for the treatment of moderate to severe atopic dermatitis in adults and adolescents 12 years and older who are candidates for systemic therapy.
Ulcerative colitis
RINVOQ is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.
Treatment of ulcerative colitis
Treatment of Crohn's disease
Treatment of vasculitides
Treatment of vasculitides
Treatment of atopic dermatitis
Treatment of chronic idiopathic arthritis (including rheumatoid arthritis , psoriatic arthritis , spondyloarthritis and juvenile idiopathic arthritis )
Upadacitinib is a DMARD that works by inhibiting the Janus Kinases (JAKs), which are essential downstream cell signalling mediators of pro-inflammatory cytokines. It is believed that these pro-inflammatory cytokines play a role in many autoimmune inflammatory conditions, such as rheumatoid arthritis. In clinical trials, upadacitinib decreased the activity of pro-inflammatory interleukins, transiently increased the levels of lymphocytes, and insignificantly decreased the levels of immunoglobulins from the baseline.
Antirheumatic Agents
Drugs that are used to treat RHEUMATOID ARTHRITIS. (See all compounds classified as Antirheumatic Agents.)
Janus Kinase Inhibitors
Agents that inhibit JANUS KINASES. (See all compounds classified as Janus Kinase Inhibitors.)
L04AA
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AA - Selective immunosuppressants
L04AA44 - Upadacitinib
Absorption
Upadacitinib displays a dose-proportional pharmacokinetic profile over the therapeutic dose range. Following oral administration, the median time to reach Cmax (Tmax) ranges from 2 to 4 hours. The steady-state plasma concentrations of upadacitinib are reached within 4 days following multiple once-daily administrations, with minimal accumulation. Food intake has no clinically relevant effect on the AUC, Cmax, and Cmin of upadacitinib from the extended-release formulation.
Route of Elimination
Following administration of a single radio-labelled dose from the immediate-release formulation, approximately 53% of the total dose was excreted in the feces where 38% of the excreted dose was an unchanged parent drug. About 43% of the total dose was excreted in the urine, where 24% of that dose was in the unchanged parent drug form. Approximately 34% of the total dose of upadacitinib dose was excreted as metabolites.
Volume of Distribution
The volume of distribution of upadacitinib in a patient with rheumatoid arthritis and a body weight of 74 kg is estimated to be 224 L following oral administration of an extended-release formula. In a pharmacokinetic study consisting of healthy volunteers receiving the extended-release formulation, the steady-state volume of distribution was 294 L. Upadacitinib partitions similarly between plasma and blood cellular components with a blood to plasma ratio of 1.0.
Clearance
The apparent oral clearance of upadacitinib in healthy volunteers receiving the extended-release formulation was 53.7 L/h.
Upadacitinib predominantly undergoes CYP3A4-mediated metabolism; however, upadacitinib is a nonsensitive substrate of CYP3A4. It is also metabolized by CYP2D6 to a lesser extent. In a human radio-labelled study, about 79% of the total plasma radioactivity accounted for the parent drug, and about 13% of the total plasma radioactivity accounted for the main metabolite produced from mono-oxidation, followed by glucuronidation. There are no known active metabolites of upadacitinib.
The mean terminal elimination half-life of upadacitinib ranged from 8 to 14 hours following administration of the extended-release formulation. In clinical trials, approximately 90% of upadacitinib in the systemic circulation was eliminated within 24 hours of dosing.
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease that involves the interplay of several mediators, including the immune cells (mainly T- and B-lymphocytes) and pro-inflammatory cytokines, such as the tumour necrosis factor (TNF), transforming growth factor (TGF), and interleukin 6 (IL-6). The Janus Kinase (JAK) family plays an essential role in the normal physiological functions (such as erythropoiesis), but also the signalling of pro-inflammatory cytokines that are implicated in many immune-mediated diseases. The JAK family consists of four isoforms (JAK1, JAK2, JAK3, and Tyrosine Kinase 2) that each interacts with different cytokine receptors and uniquely associates with the intracellular domains of Type I/II cytokine receptors. JAK1 is primarily involved in the signalling transduction pathways of IL-6, IFN and the common -chain cytokines, including IL-2 and IL-15. IL-6 has been closely studied in particular, as it is a major cytokine involved in B- and T-cell differentiation and the acute phase response in inflammation. Upon interaction of cytokines with their cytokine receptors, the JAKs mediate the JAK-STAT signal transduction pathway in response to receptor activation. JAKs are tyrosine kinases that cause phosphorylation of several proteins, including cytokine receptors and JAKs themselves. Phosphorylation of JAKs promotes the phosphorylation and activation of the signalling molecules called STATs, leading to their nuclear translocation, binding to DNA promoters, and target gene transcription. JAK1-mediated signalling pathways ultimately promote pro-inflammatory events, such as increased proliferation and survival of immune cells, T cell differentiation, and macrophage activation. Upadacitinib is a selective JAK1 inhibitor that has a negligible effect on JAK3, leading to an improved drug safety profile. Upadacitinib blocks the cellular processes that contribute to the inflammatory conditions in rheumatoid arthritis. In human leukocytes cellular assays, upadacitinib inhibited JAK1/3-induced phosphorylation of STAT3/5 mediated by IL-6/7.
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39979
Submission : 2024-07-30
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2023-04-24
Pay. Date : 2023-02-21
DMF Number : 38095
Submission : 2023-02-22
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40355
Submission : 2024-08-28
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38427
Submission : 2023-06-15
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2022-08-16
Pay. Date : 2022-06-15
DMF Number : 37149
Submission : 2022-06-01
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39581
Submission : 2024-03-12
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2023-07-17
Pay. Date : 2023-04-25
DMF Number : 38153
Submission : 2023-03-26
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38051
Submission : 2023-02-09
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40344
Submission : 2024-08-19
Status : Active
Type : II

USDMF
GDUFA
DMF Review : Complete
Rev. Date : 2023-07-05
Pay. Date : 2023-05-15
DMF Number : 38344
Submission : 2023-05-20
Status : Active
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Rinvoq (upadacitinib) is a FDA approved selective JAK inhibitor that is being investigated in adult patients with giant cell arteritis in a Phase 3 clinical trial.
Lead Product(s): Upadacitinib,Corticosteroid
Therapeutic Area: Immunology Brand Name: Rinvoq
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 08, 2025
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Upadacitinib,Corticosteroid
Therapeutic Area : Immunology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
AbbVie Wins EU Nod for RINVOQ in Adults with Giant Cell Arteritis
Details : Rinvoq (upadacitinib) is a FDA approved selective JAK inhibitor that is being investigated in adult patients with giant cell arteritis in a Phase 3 clinical trial.
Product Name : Rinvoq
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
April 08, 2025
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Rinvoq (upadacitinib) is a FDA approved selective JAK inhibitor that is being investigated in adult patients with giant cell arteritis in a Phase 3 clinical trial.
Lead Product(s): Upadacitinib,Corticosteroid
Therapeutic Area: Immunology Brand Name: Rinvoq
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 28, 2025
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Upadacitinib,Corticosteroid
Therapeutic Area : Immunology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
AbbVie gets Positive CHMP Opinion for RINVOQ® in Giant Cell Arteritis Treatment
Details : Rinvoq (upadacitinib) is a FDA approved selective JAK inhibitor that is being investigated in adult patients with giant cell arteritis in a Phase 3 clinical trial.
Product Name : Rinvoq
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
February 28, 2025
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Rinvoq (upadacitinib) is a FDA approved selective JAK inhibitor that is being investigated in adult patients with giant cell arteritis as a Phase 3 clinical trial.
Lead Product(s): Upadacitinib
Therapeutic Area: Immunology Brand Name: Rinvoq
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 23, 2024
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Upadacitinib
Therapeutic Area : Immunology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
AbbVie’s Phase 3 SELECT-GCA Study of Upadacitinib Shows Positive Results in GCA
Details : Rinvoq (upadacitinib) is a FDA approved selective JAK inhibitor that is being investigated in adult patients with giant cell arteritis as a Phase 3 clinical trial.
Product Name : Rinvoq
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
April 23, 2024
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Rinvoq (upadacitinib) is a janus kinase inhibitor, small molecule drug candidate which is being evaluated for the treatment of giant cell arteritis.
Lead Product(s): Upadacitinib,Undisclosed
Therapeutic Area: Immunology Brand Name: Rinvoq
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 18, 2024
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Upadacitinib,Undisclosed
Therapeutic Area : Immunology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Phase 3 SELECT-GCA Study of Upadacitinib Shows Positive Results for Giant Cell Arteritis
Details : Rinvoq (upadacitinib) is a janus kinase inhibitor, small molecule drug candidate which is being evaluated for the treatment of giant cell arteritis.
Product Name : Rinvoq
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
April 18, 2024
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Rinvoq (upadacitinib), a Janus kinase (JAK) inhibitor that interferes with the JAK-STAT signaling pathway, being developed for the treatment in adults with non-segmental vitiligo.
Lead Product(s): Upadacitinib
Therapeutic Area: Dermatology Brand Name: Rinvoq
Study Phase: Phase IIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 10, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Upadacitinib
Therapeutic Area : Dermatology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
AbbVie Announces Upadacitinib (RINVOQ®) Met the Primary Endpoint in Phase 2 Clinical Trial of Vit...
Details : Rinvoq (upadacitinib), a Janus kinase (JAK) inhibitor that interferes with the JAK-STAT signaling pathway, being developed for the treatment in adults with non-segmental vitiligo.
Product Name : Rinvoq
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
December 10, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Rinvoq (upadacitinib), a Janus kinase (JAK) inhibitor that interferes with the JAK-STAT signaling pathway, indicated for the treatment of adults with moderately to severely active rheumatoid arthritis, active psoriatic arthritis and atopic dermatitis.
Lead Product(s): Upadacitinib
Therapeutic Area: Immunology Brand Name: Rinvoq
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 25, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Upadacitinib
Therapeutic Area : Immunology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Health Canada Approves AbbVie's RINVOQ® (upadacitinib) for the Treatment of Adults with Moderatel...
Details : Rinvoq (upadacitinib), a Janus kinase (JAK) inhibitor that interferes with the JAK-STAT signaling pathway, indicated for the treatment of adults with moderately to severely active rheumatoid arthritis, active psoriatic arthritis and atopic dermatitis.
Product Name : Rinvoq
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
July 25, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Rinvoq (upadacitinib) is a selective JAK inhibitor that is being studied in several immune-mediated inflammatory diseases including, hidradenitis suppurativa in the Phase 3 clinical trial.
Lead Product(s): Upadacitinib
Therapeutic Area: Dermatology Brand Name: Rinvoq
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 24, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Upadacitinib
Therapeutic Area : Dermatology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Rinvoq (upadacitinib) is a selective JAK inhibitor that is being studied in several immune-mediated inflammatory diseases including, hidradenitis suppurativa in the Phase 3 clinical trial.
Product Name : Rinvoq
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
July 24, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
ABBV-599 is a novel fixed-dose combination of the Bruton's tyrosine kinase (BTK) inhibitor elsubrutinib and the Janus kinase (JAK) inhibitor upadacitinib under investigation for the treatment of systemic lupus erythematosus.
Lead Product(s): Elsubrutinib,Upadacitinib
Therapeutic Area: Immunology Brand Name: ABBV-599
Study Phase: Phase IIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 31, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elsubrutinib,Upadacitinib
Therapeutic Area : Immunology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Phase 2 Study of Upadacitinib (RINVOQ®) Alone or as a Combination Therapy Meets Primary and Key S...
Details : ABBV-599 is a novel fixed-dose combination of the Bruton's tyrosine kinase (BTK) inhibitor elsubrutinib and the Janus kinase (JAK) inhibitor upadacitinib under investigation for the treatment of systemic lupus erythematosus.
Product Name : ABBV-599
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
May 31, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Rinvoq (upadacitinib) is a JAK inhibitor, involved in the process of immune-mediated inflammatory diseases. It has been approved by USFDA for the treatment of moderately to severely active crohn's disease.
Lead Product(s): Upadacitinib
Therapeutic Area: Immunology Brand Name: Rinvoq
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 18, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Upadacitinib
Therapeutic Area : Immunology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
U.S. FDA Approves RINVOQ® (upadacitinib) as a Once-Daily Pill for Moderately to Severely Active C...
Details : Rinvoq (upadacitinib) is a JAK inhibitor, involved in the process of immune-mediated inflammatory diseases. It has been approved by USFDA for the treatment of moderately to severely active crohn's disease.
Product Name : Rinvoq
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
May 18, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Rinvoq (upadacitinib) is a JAK inhibitor, involved in the process of immune-mediated inflammatory diseases. It has been approved in EU for the treatment of moderately to severely active crohn's disease.
Lead Product(s): Upadacitinib
Therapeutic Area: Immunology Brand Name: Rinvoq
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 17, 2023
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Upadacitinib
Therapeutic Area : Immunology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
AbbVie Announces European Commission Approval of RINVOQ® (upadacitinib) for the Treatment of Mode...
Details : Rinvoq (upadacitinib) is a JAK inhibitor, involved in the process of immune-mediated inflammatory diseases. It has been approved in EU for the treatment of moderately to severely active crohn's disease.
Product Name : Rinvoq
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
April 17, 2023
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]CAS Number : 5034-06-0
End Use API : Upadacitinib
About The Company : Cohance Lifesciences is a leading CDMO and API platform, offering products and services across all phases of a molecule’s lifecycle from development to commer...
(3R,4S)-3-(2-Bromoacetyl)-4- ethyl-1-pyrrolidineca...
CAS Number : 1428243-26-8
End Use API : Upadacitinib
About The Company : Shandong Chenghui Shuangda Pharmaceutical Co., Ltd. is specialized in R&D and production of APIs and advanced intermediates. With 22 years of production experie...
tert-butyl 5-tosyl-5H-pyrrolo[2,3- b]pyrazin-2-yl...
CAS Number : 1201187-44-1
End Use API : Upadacitinib
About The Company : Shandong Chenghui Shuangda Pharmaceutical Co., Ltd. is specialized in R&D and production of APIs and advanced intermediates. With 22 years of production experie...
8-((3R,4S)-4-ethylpyrrolidin-3-yl)- 3-tosyl-3H-imi...
CAS Number : 1428243-28-0
End Use API : Upadacitinib
About The Company : Shandong Chenghui Shuangda Pharmaceutical Co., Ltd. is specialized in R&D and production of APIs and advanced intermediates. With 22 years of production experie...
CAS Number : 5034-06-0
End Use API : Upadacitinib
About The Company : Since 2010, our dedication to our clientele has been unwavering in addressing pharmaceutical hurdles, regardless of scale. We prioritize integrity, responsivene...
2-bromo-5-(p-tolylsulfonyl) pyrrolo[2,3-b] pyrazin...
CAS Number : 1201186-54-0
End Use API : Upadacitinib
About The Company : Aarti Pharmalab, earlier the pharma division of Aarti Industries, is a leading Indian manufacturer of APIs. It has dedicated facilities to manufacture HPAPIs, c...
N-[5-[(4-methylphenyl)sulfonyl]-5H-pyrrolo[2,3- b]...
CAS Number : 1201187-44-1
End Use API : Upadacitinib
About The Company : Aarti Pharmalab, earlier the pharma division of Aarti Industries, is a leading Indian manufacturer of APIs. It has dedicated facilities to manufacture HPAPIs, c...
(3R,4S)-3-(2-Bromoacetyl)-4-ethyl-1-pyrrolidinecar...
CAS Number : 1428243-26-8
End Use API : Upadacitinib
About The Company : Jinan Tantu Chemicals Co., Ltd. operates as a Contract Development and Manufacturing Organization (CDMO) that serves pharmaceutical companies worldwide. Our cor...
N-[5-[(4-Methylphenyl)sulfonyl]-5H-pyrrolo[2,3-b]p...
CAS Number : 1201187-44-1
End Use API : Upadacitinib
About The Company : Jinan Tantu Chemicals Co., Ltd. operates as a Contract Development and Manufacturing Organization (CDMO) that serves pharmaceutical companies worldwide. Our cor...
3,7-Dihydro-4H-Pyrrolo[2,3-d] Pyrimidin-4-One
CAS Number : CAS-3680-71-5
End Use API : Upadacitinib
About The Company : Almelo are the industry leaders in manufacturing advanced intermediates, Active Pharmaceutical Ingredients (APIs) and specialty fine chemicals. With a diverse p...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
30
PharmaCompass offers a list of Upadacitinib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Upadacitinib manufacturer or Upadacitinib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Upadacitinib manufacturer or Upadacitinib supplier.
PharmaCompass also assists you with knowing the Upadacitinib API Price utilized in the formulation of products. Upadacitinib API Price is not always fixed or binding as the Upadacitinib Price is obtained through a variety of data sources. The Upadacitinib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Upadacitinib manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Upadacitinib, including repackagers and relabelers. The FDA regulates Upadacitinib manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Upadacitinib API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Upadacitinib manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Upadacitinib supplier is an individual or a company that provides Upadacitinib active pharmaceutical ingredient (API) or Upadacitinib finished formulations upon request. The Upadacitinib suppliers may include Upadacitinib API manufacturers, exporters, distributors and traders.
click here to find a list of Upadacitinib suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Upadacitinib DMF (Drug Master File) is a document detailing the whole manufacturing process of Upadacitinib active pharmaceutical ingredient (API) in detail. Different forms of Upadacitinib DMFs exist exist since differing nations have different regulations, such as Upadacitinib USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Upadacitinib DMF submitted to regulatory agencies in the US is known as a USDMF. Upadacitinib USDMF includes data on Upadacitinib's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Upadacitinib USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Upadacitinib suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Upadacitinib Drug Master File in Korea (Upadacitinib KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Upadacitinib. The MFDS reviews the Upadacitinib KDMF as part of the drug registration process and uses the information provided in the Upadacitinib KDMF to evaluate the safety and efficacy of the drug.
After submitting a Upadacitinib KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Upadacitinib API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Upadacitinib suppliers with KDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Upadacitinib as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Upadacitinib API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Upadacitinib as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Upadacitinib and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Upadacitinib NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Upadacitinib suppliers with NDC on PharmaCompass.
Upadacitinib Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Upadacitinib GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Upadacitinib GMP manufacturer or Upadacitinib GMP API supplier for your needs.
A Upadacitinib CoA (Certificate of Analysis) is a formal document that attests to Upadacitinib's compliance with Upadacitinib specifications and serves as a tool for batch-level quality control.
Upadacitinib CoA mostly includes findings from lab analyses of a specific batch. For each Upadacitinib CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Upadacitinib may be tested according to a variety of international standards, such as European Pharmacopoeia (Upadacitinib EP), Upadacitinib JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Upadacitinib USP).
