
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
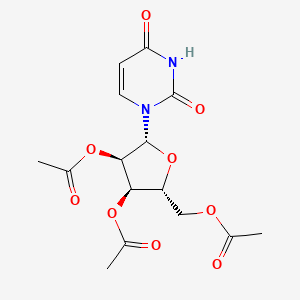

1. 2',3',5'-tri-o-acetyluridine
2. Pn 401
3. Pn-401
4. Pn401
5. Rg-2133
6. Rg2133
7. Triacetyluridine
8. Xuriden
1. 4105-38-8
2. 2',3',5'-tri-o-acetyluridine
3. Triacetyluridine
4. Vistonuridine
5. Pn401
6. 2',3',5'-triacetyluridine
7. Xuriden
8. Tri-o-acetyluridine
9. Uridine 2',3',5'-triacetate
10. Vistogard
11. (2r,3r,4r,5r)-2-(acetoxymethyl)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2h)-yl)tetrahydrofuran-3,4-diyl Diacetate
12. Triacetyl Uridine
13. Uridine, 2',3',5'-triacetate
14. Pn 401
15. Pn-401
16. Rg2133
17. Rg 2133
18. Rg-2133
19. Mfcd00023795
20. 2wp61f175m
21. Unii-2wp61f175m
22. Uridinetriacetate
23. Uridine Triacetate [usan:inn]
24. Uridine-triacetate
25. Vistogard (tn)
26. Einecs 223-881-5
27. Xuriden (tn)
28. Uridine, 2,3,5-triacetate
29. Mls006009982
30. Schembl871011
31. Uridine Triacetate [mi]
32. Chembl2107381
33. Uridine Triacetate (usan/inn)
34. Uridine Triacetate [inn]
35. Chebi:90914
36. Dtxsid40961409
37. Uridine Triacetate [usan]
38. [(2r,3r,4r,5s)-3,4-diacetyloxy-5-(2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methyl Acetate
39. Cs-d1799
40. Uridine Triacetate [who-dd]
41. Zinc3843198
42. Nsc788948
43. S6484
44. Akos015964563
45. Db09144
46. Nsc-788948
47. [(2r,3r,4r,5r)-3,4-diacetyloxy-5-(2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methyl Acetate
48. Uridine Triacetate [orange Book]
49. Hy-14905
50. Smr004701034
51. Sy004945
52. 2',3',5'-tri-o-acetyluridine, >=98%
53. D09985
54. 105t388
55. A825419
56. J-700012
57. Q22075857
58. Uridine 2',3',5'-triacetate;2',3',5'-triacetyluridine
59. 2 Inverted Exclamation Mark ,3 Inverted Exclamation Mark ,5 Inverted Exclamation Mark -triacetyluridine
| Molecular Weight | 370.31 g/mol |
|---|---|
| Molecular Formula | C15H18N2O9 |
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 8 |
| Exact Mass | 370.10123016 g/mol |
| Monoisotopic Mass | 370.10123016 g/mol |
| Topological Polar Surface Area | 138 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 660 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Marketed as the product Xuriden (FDA), uridine triacetate is indicated for the treatment of hereditary orotic aciduria. Marketed as the product Vistogard (FDA), uridine triacetate is indicated for the emergency treatment of adult and pediatric patients in the following situations: following a fluorouracil or capecitabine overdose regardless of the presence of symptoms; or who exhibit early-onset, severe or life-threatening toxicity affecting the cardiac or central nervous system, and/or early-onset, unusually severe adverse reactions (e.g., gastrointestinal toxicity and/or neutropenia) within 96 hours following the end of fluorouracil or capecitabine administration.
FDA Label
A - Alimentary tract and metabolism
A16 - Other alimentary tract and metabolism products
A16A - Other alimentary tract and metabolism products
A16AX - Various alimentary tract and metabolism products
A16AX13 - Uridine triacetate
Absorption
Maximum concentrations of uridine in plasma following oral administration are generally achieved within 2 to 3 hours.
Route of Elimination
Uridine can be excreted via the kidneys, but is also metabolized by normal pyrimidine catabolic pathways present in most tissues.
Volume of Distribution
Circulating uridine is taken up into mammalian cells via specific nucleoside transporters, and also crosses the blood brain barrier.
Following oral administration, uridine triacetate is deacetylated by nonspecific esterases present throughout the body, yielding uridine in the circulation.
2 to 2.5 hours
Uridine triacetate is a synthetic uridine pro-drug that is converted to uridine in vivo. When used for the treatment or prevention of toxicity associated with fluorouracil and other antimetabolites, uridine triacetate is utilized for its ability to compete with 5-fluorouracil (5-FU) metabolites for incorporation into the genetic material of non-cancerous cells. It reduces toxicity and cell-death associated with two cytotoxic intermediates: 5-fluoro-2'-deoxyuridine-5'-monophosphate (FdUMP) and 5-fluorouridine triphosphate (FUTP). By pre-administering with uridine (as the prodrug uridine triacetate), higher doses of 5-FU can be given allowing for improved efficacy and a reduction in toxic side effects such as neutropenia, mucositis, diarrhea, and handfoot syndrome. Uridine triacetate is also used for replacement therapy in the treatment of hereditary orotic aciduria, also known as uridine monophosphate synthase (UMPS) deficiency. As a result of UMPS deficiency, patients experience a systemic deficiency of pyrimidine nucleotides, accounting for most symptoms of the disease. Additionally, orotic acid from the de novo pyrimidine pathway that cannot be converted to UMP is excreted in the urine, accounting for the common name of the disorder, orotic aciduria. Furthermore, orotic acid crystals in the urine can cause episodes of obstructive uropathy. When administered as the prodrug uridine triacetate, uridine can be used by essentially all cells to make uridine nucleotides, which compensates for the genetic deficiency in synthesis in patients with hereditary orotic aciduria.
Related Excipient Companies

Excipients by Applications
Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
20
PharmaCompass offers a list of Triacetyluridine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Triacetyluridine manufacturer or Triacetyluridine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Triacetyluridine manufacturer or Triacetyluridine supplier.
PharmaCompass also assists you with knowing the Triacetyluridine API Price utilized in the formulation of products. Triacetyluridine API Price is not always fixed or binding as the Triacetyluridine Price is obtained through a variety of data sources. The Triacetyluridine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Uridine triacetate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Uridine triacetate, including repackagers and relabelers. The FDA regulates Uridine triacetate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Uridine triacetate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Uridine triacetate supplier is an individual or a company that provides Uridine triacetate active pharmaceutical ingredient (API) or Uridine triacetate finished formulations upon request. The Uridine triacetate suppliers may include Uridine triacetate API manufacturers, exporters, distributors and traders.
click here to find a list of Uridine triacetate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Uridine triacetate DMF (Drug Master File) is a document detailing the whole manufacturing process of Uridine triacetate active pharmaceutical ingredient (API) in detail. Different forms of Uridine triacetate DMFs exist exist since differing nations have different regulations, such as Uridine triacetate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Uridine triacetate DMF submitted to regulatory agencies in the US is known as a USDMF. Uridine triacetate USDMF includes data on Uridine triacetate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Uridine triacetate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Uridine triacetate suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Uridine triacetate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Uridine triacetate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Uridine triacetate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Uridine triacetate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Uridine triacetate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Uridine triacetate suppliers with NDC on PharmaCompass.
Uridine triacetate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Uridine triacetate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Uridine triacetate GMP manufacturer or Uridine triacetate GMP API supplier for your needs.
A Uridine triacetate CoA (Certificate of Analysis) is a formal document that attests to Uridine triacetate's compliance with Uridine triacetate specifications and serves as a tool for batch-level quality control.
Uridine triacetate CoA mostly includes findings from lab analyses of a specific batch. For each Uridine triacetate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Uridine triacetate may be tested according to a variety of international standards, such as European Pharmacopoeia (Uridine triacetate EP), Uridine triacetate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Uridine triacetate USP).
