
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
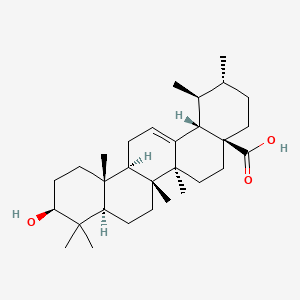

1. (+)-ursolic Acid
2. (3 Beta)-3-hydroxyurs-12-en-28-oic Acid
3. 3-epi-ursolic Acid
4. 3-epiursolic Acid
5. 3alpha-ursolic Acid
6. 3beta-ursolic Acid
7. Merotaine
8. Olean-12-en-28-oic Acid, 3-hydroxy-, Sodium Salt (1:1), (3beta)-
9. Sodium Oleanolate
10. Ursolic Acid Monosodium Salt
11. Ursolic Acid Sodium Salt
12. Ursolic Acid, (3beta)-isomer, 2-(14)c-labeled
13. Ursolic Acid, (3beta)-isomer, Monopotassium Salt
1. 77-52-1
2. Prunol
3. Malol
4. Urson
5. 3beta-hydroxyurs-12-en-28-oic Acid
6. (3beta)-3-hydroxyurs-12-en-28-oic Acid
7. Micromerol
8. Ursolic-acid
9. Nsc-4060
10. Nsc 167406
11. (+)-ursolic Acid
12. Chebi:9908
13. Chembl169
14. Nsc-167406
15. P3m2575f3f
16. .beta.-ursolic Acid
17. (1s,2r,4as,6ar,6as,6br,8ar,10s,12ar,14bs)-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydro-1h-picene-4a-carboxylic Acid
18. 3b-hydroxyurs-12-en-28-oic Acid
19. Nsc 4060
20. Bungeolic Acid
21. 3beta-hydroxy-12-ursen-28-ic Acid
22. Nsc4060
23. Mfcd00009621
24. Smr000445681
25. Sr-01000779684
26. Einecs 201-034-0
27. Urs-12-en-28-oic Acid, 3-hydroxy-, (3.beta.)-
28. Tnp00103
29. Unii-p3m2575f3f
30. Uosolic Acid
31. Ai3-03109
32. Hsdb 7685
33. 6q5
34. Urs-12-en-28-oic Acid, 3-hydroxy-, (3beta)-
35. Urs-12-en-28-oic Acid, 3beta-hydroxy-
36. Prestwick3_000089
37. Ursolic Acid, >=90%
38. Ursolic Acid [mi]
39. Ursolic Acid [hsdb]
40. Ursolic Acid [inci]
41. Schembl70205
42. Bspbio_000018
43. Mls000728569
44. Mls002154196
45. Mls002207073
46. Ursolic Acid [usp-rs]
47. Ursolic Acid [who-dd]
48. Bpbio1_000020
49. N-ethylhydroxylaminehydrochloride
50. Dtxsid70883221
51. Ursolic Acid, Analytical Standard
52. 3beta-hydroxyurs-12-en-28-oate
53. Hms2095a20
54. Hms2231p19
55. (1s,2r,4as,6as,6br,8ar,10s,12ar,12br,14bs)-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylic Acid
56. 3beta-hydroxy-urs-12-en-28-oate
57. Hy-n0140
58. Zinc3978827
59. Bdbm50148911
60. 3beta-hydroxy-12-ursen-28-oic Acid
61. Akos005228010
62. Akos016023773
63. 3.beta.-hydroxy-urs-12-en-28-oate
64. Ccg-208282
65. Cs-3799
66. Db15588
67. Lmpr0106180007
68. (3beta)-3-hydroxyurs-12-en-28-oate
69. 3beta-hydroxy-urs-12-en-28-oic Acid
70. (3beta)-3-hydroxy-urs-12-en-28-oate
71. As-35119
72. 3.beta.-hydroxy-urs-12-en-28-oic Acid
73. (3beta)-3-hydroxy-urs-12-en-28-oic Acid
74. Ab00513802
75. N1823
76. U0065
77. C08988
78. 009u621
79. A839123
80. Q416260
81. Q-201916
82. Sr-01000779684-4
83. Sr-01000779684-5
84. (3.beta.)-3-hydroxyurs-12-en-28-oic Acid
85. Brd-k68185022-001-02-3
86. Brd-k68185022-001-14-8
87. Ursolic Acid, Primary Pharmaceutical Reference Standard
88. Af479d19-631e-48f1-8aba-fb2a806046fa
89. Ursolic Acid (constituent Of Holy Basil Leaf) [dsc]
90. (3beta,5beta,18alpha,20beta)-3-hydroxyurs-12-en-28-oic Acid
91. Ursolic Acid, European Pharmacopoeia (ep) Reference Standard
92. Ursolic Acid, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 456.7 g/mol |
|---|---|
| Molecular Formula | C30H48O3 |
| XLogP3 | 7.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 456.36034539 g/mol |
| Monoisotopic Mass | 456.36034539 g/mol |
| Topological Polar Surface Area | 57.5 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 874 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL/ The methanol extract (ME) and the n-butanol fractions of methanolic extract of Alstonia macrophylla Wall ex A. DC leaves were investigated on the forward motility (FM) of mammalian (goat and human) spermatozoa. The ME at 600 ug/mL as well as fraction B at 100 ug/mL concentrations showed marked inhibition of sperm FM in both goat and human species ... .. Approximately 60-80% of the goat spermatozoa lost their FM when treated with 600 ug/mL of ME and 100 ug/mL of fraction B. At 100 ug/mL concentration, fraction B showed 90% loss of FM in human spermatozoa, while fraction B at 400 ug/mL concentration showed complete inhibition of sperm FM at 0 min. The inhibitory activity of fraction B increases with increasing concentration in a dose-dependent manner. ... Fraction B (ursolic acid), a pentacyclic triterpene, has the potential of sperm motility inhibition and can serve as a topical vaginal contraceptive.
PMID:15854639 Chattopadhyay D et al; Contraception 71 (5): 372-8 (2005)
Both oleanolic acid and ursolic acid are effective in protecting against chemically induced liver injury in laboratory animals. Oleanolic acid has been marketed in China as an oral drug for human liver disorders.
PMID:8847885 Liu J; J Ethnopharmacol 49 (2): 57-68 (1995)
Antineoplastic Agents, Phytogenic
Agents obtained from higher plants that have demonstrable cytostatic or antineoplastic activity. (See all compounds classified as Antineoplastic Agents, Phytogenic.)
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
/The authors/ previously reported that ursolic acid, a pentacyclic triterpene acid, inhibited the invasion of HT1080 human fibrosarcoma cells by reducing the expression of matrix metalloproteinase-9. Since the chemical structure of ursolic acid is very similar to that of dexamethasone, a synthetic glucocorticoid, ... whether ursolic acid acts through the glucocorticoid receptor /was investigated/. The expression of matrix metalloproteinase-9 is thought to be regulated similarly with matrix metalloproteinase-1 and matrix metalloproteinase-3 as containing common 2-O-tetradecanoylphorbol-acetate responsible region, where AP-1 proteins can bind. Dexamethasone has been studied to repress the 2-O-tetradecanoylphorbol-acetate-induced expression of matrix metalloproteinase-1 and matrix metalloproteinase-3 through a glucocorticoid receptor-mediated manner. In Northern blot analysis, we found that ursolic acid reduced the expression of matrix metalloproteinase-1 and matrix metalloproteinase-3 induced by 2-O-tetradecanoylphorbol-acetate. Similarly, ursolic acid down-regulated 2-O-tetradecanoylphorbol-acetate-induction of matrix metalloproteinase-9 gene in the same manner of dexamethasone. RU486, a potent glucocorticoid receptor antagonist, was used for identifying that ursolic acid-induced down-regulation of matrix metalloproteinase-9 expression is mediated by its binding to glucocorticoid receptor. The effect of ursolic acid on the matrix metalloproteinase-9 expression was blocked by RU486, suggesting that ursolic acid acts via a glucocorticoid receptor in the regulation of matrix metalloproteinase-9. Western blot analysis and immunocytochemistry showed that ursolic acid increased glucocorticoid receptor fraction in the nucleus, although it decreased the synthesis of glucocorticoid receptor mRNA. In addition, ursolic acid did not decrease the expression of c-jun and DNA-binding activity of AP-1 to its cognate sequences. Taken together, ... ursolic acid may induce the repression of matrix metalloproteinase-9 by stimulating the nuclear translocation of glucocorticoid receptor, and the translocated glucocorticoid receptor probably down-modulating the trans-activating function of AP-1 to 2-O-tetradecanoylphorbol-acetate responsible element of matrix metalloproteinase-9 promoter region.
PMID:9488041 Cha HJ et al; Oncogene 16 (6): 771-8 (1998)
... Ursolic acid suppressed NF-kappaB activation induced by various carcinogens including tumor necrosis factor (TNF), phorbol ester, okadaic acid, H2O2, and cigarette smoke. These effects were not cell type specific. Ursolic acid inhibited DNA binding of NF-kappaB consisting of p50 and p65. Ursolic acid inhibited IkappaBalpha degradation, IkappaBalpha phosphorylation, IkappaBalpha kinase activation, p65 phosphorylation, p65 nuclear translocation, and NF-kappaB-dependent reporter gene expression. Ursolic acid also inhibited NF-kappaB-dependent reporter gene expression activated by TNF receptor, TNF receptor-associated death domain, TNF receptor-associated factor, NF-kappaB-inducing kinase, IkappaBalpha kinase, and p65. The inhibition of NF-kappaB activation correlated with suppression of NF-kappaB-dependent cyclin D1, cyclooxygenase 2, and matrix metalloproteinase 9 expression. Thus, overall, /the/ results indicate that ursolic acid inhibits IkappaBalpha kinase and p65 phosphorylation, leading to the suppression of NF-kappaB activation induced by various carcinogens. These actions of ursolic acid may mediate its antitumorigenic and chemosensitizing effects.
PMID:12907607 Shishodia S et al; Cancer Res 63 (15): 4375-83 (2003)
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
24
PharmaCompass offers a list of Ursolic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ursolic Acid manufacturer or Ursolic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ursolic Acid manufacturer or Ursolic Acid supplier.
PharmaCompass also assists you with knowing the Ursolic Acid API Price utilized in the formulation of products. Ursolic Acid API Price is not always fixed or binding as the Ursolic Acid Price is obtained through a variety of data sources. The Ursolic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ursolic Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ursolic Acid, including repackagers and relabelers. The FDA regulates Ursolic Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ursolic Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ursolic Acid manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ursolic Acid supplier is an individual or a company that provides Ursolic Acid active pharmaceutical ingredient (API) or Ursolic Acid finished formulations upon request. The Ursolic Acid suppliers may include Ursolic Acid API manufacturers, exporters, distributors and traders.
click here to find a list of Ursolic Acid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Ursolic Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ursolic Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ursolic Acid GMP manufacturer or Ursolic Acid GMP API supplier for your needs.
A Ursolic Acid CoA (Certificate of Analysis) is a formal document that attests to Ursolic Acid's compliance with Ursolic Acid specifications and serves as a tool for batch-level quality control.
Ursolic Acid CoA mostly includes findings from lab analyses of a specific batch. For each Ursolic Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ursolic Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Ursolic Acid EP), Ursolic Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ursolic Acid USP).

