
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
API
0
FDF
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Weekly News Recap #Phispers
US Medicaid
NA
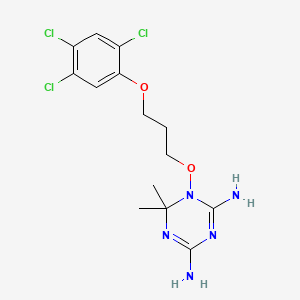

1. 4,6-diamino-(1,2-dihydro)-2,2-dimethyl-1-(2,4,5-trichlorophenoxypropyloxy)-1,3,5-triazine.hcl
2. Brl 51084
3. Brl 6231
4. Brl 6231hydrochloride
5. Brl 6231mono-hydrobromide
6. Brl-51084
7. Brl-6231
8. Unspecified Hcl Of Brl 6231
9. Wr 99210
10. Wr-99210
11. Wr99210-hcl
1. Wr99210
2. 47326-86-3
3. Wr-99210
4. 1,3,5-triazine-2,4-diamine, 1,6-dihydro-6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]-
5. Brl 6231
6. Chembl129788
7. Wr 99210
8. 6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]-1,3,5-triazine-2,4-diamine
9. 1,6-dihydro-6,6-dimethyl-1-(3-(2,4,5-trichlorophenoxy)-propoxy)-1,3,5-triazine-2 ,4-diamine
10. 1,6-dihydro-6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]-1,3,5-triazine-2,4-diamine
11. 6,6-dimethyl-1-(3-(2,4,5-trichlorophenoxy)propoxy)-1,6-dihydro-1,3,5-triazine-2,4-diamine
12. 1,3,5-triazine-2,4-diamine, 1,6-dihydro-6,6-dimethyl-1-(3-(2,4,5-trichlorophenoxy)propoxy)-
13. Wra
14. Brn 0629517
15. Wr-99,210
16. 1-(2',4',5'-trichlorophenoxypropoxy)-1,2-dihydro-2,2-dimethyl-4,6-diamino-s-triazine
17. 1,6-dihydro-6,6-dimethyl-1-(3-(2,4,5-trichlorophenoxy)propoxy)-1,3,5-triazine-2,4-diamine, Monohydrochloride
18. Schembl8635467
19. Bdbm18793
20. Dtxsid30952947
21. Xba32686
22. Zinc3581056
23. Wr-99209 (*hydrogen Bromide*)
24. Brl 6231 (*monohydrogen Chloride*)
25. Db08734
26. 6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]-1,3,5-triazinane-2,4-diimine
27. 4,6-diamino-1,2-dihydro-2,2-dimethyl-1-[(2,4,5-trichlorophenoxy)propyloxy]-1,3,5-triazine
28. Hy-116387
29. Cs-0065315
30. Q27467334
31. 2,2-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]-1,3,5-triazine-4,6-diamine
32. 4-imino-6,6-dimethyl-5-[3-(2,4,5-trichlorophenoxy)propoxy]-1h-1,3,5-triazin-2-amine
| Molecular Weight | 394.7 g/mol |
|---|---|
| Molecular Formula | C14H18Cl3N5O2 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 393.052608 g/mol |
| Monoisotopic Mass | 393.052608 g/mol |
| Topological Polar Surface Area | 98.5 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 503 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Folic Acid Antagonists
Inhibitors of the enzyme, dihydrofolate reductase (TETRAHYDROFOLATE DEHYDROGENASE), which converts dihydrofolate (FH2) to tetrahydrofolate (FH4). They are frequently used in cancer chemotherapy. (From AMA, Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Folic Acid Antagonists.)
Antimalarials
Agents used in the treatment of malaria. They are usually classified on the basis of their action against plasmodia at different stages in their life cycle in the human. (From AMA, Drug Evaluations Annual, 1992, p1585) (See all compounds classified as Antimalarials.)
 Shanghai Minbiotech is the leading producer of biopharmaceuticals and a variety of high-end generic & innovative drugs.
Shanghai Minbiotech is the leading producer of biopharmaceuticals and a variety of high-end generic & innovative drugs.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : USA
Brand Name : WEZLANA
Dosage Form : INJECTABLE;INTRAVENOUS, SUBCUTANEOUS
Dosage Strength : 130MG/26ML
Packaging :
Approval Date :
Application Number : 761331
Regulatory Info :
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : USA
Brand Name : STELARA
Dosage Form : INJECTABLE; INJECTION
Dosage Strength : 45MG/0.5ML
Packaging :
Approval Date :
Application Number : 125261
Regulatory Info :
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : FINLIUS I.V.
Dosage Form : SOLUTION
Dosage Strength : 5MG/ML
Packaging :
Approval Date :
Application Number : 2537311
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : JAMTEKI
Dosage Form : SOLUTION
Dosage Strength : 90MG/1ML
Packaging :
Approval Date :
Application Number : 2543044
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : STELARA
Dosage Form : SOLUTION FOR INJECTION, PRE-FILLED SYRINGE
Dosage Strength : 90 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Stelara
Dosage Form : Inj L?s
Dosage Strength : 45mg/0.5ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Stelara
Dosage Form : Inj L?s
Dosage Strength : 90mg/ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Stelara 130 mg
Dosage Form : INJ
Dosage Strength : 130mg
Packaging : 1X1mg
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Stelara
Dosage Form :
Dosage Strength :
Packaging : 1
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Stelara
Dosage Form :
Dosage Strength :
Packaging : 1
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?