

Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
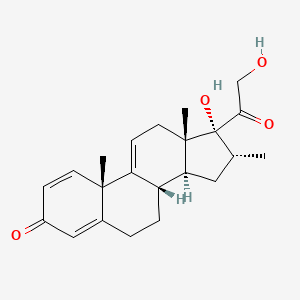

1. Vbp15 Compound
1. 13209-41-1
2. Vamorolone [usan]
3. Vbp-15 Free Alcohol
4. 17,21-dihydroxy-16alpha-methylpregna-1,4,9(11)-triene-3,20-dione
5. 16alpha-methyl-9,11-dehydro Prednisolone
6. 8xp29xmb43
7. (8s,10s,13s,14s,16r,17r)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6h-cyclopenta[a]phenanthren-3-one
8. Chembl2348780
9. Vbp15
10. (8s,10s,13s,14s,16r,17r)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,10,12,13,14,15,16,17-decahydro-3h-cyclopenta[a]phenanthren-3-one
11. Unii-8xp29xmb43
12. Vbp 15
13. Einecs 236-177-8
14. 16
15. A-methyl-9,11-dehydro Prednisolone
16. Vamorolone [inn]
17. Vamorolone (usan/inn)
18. Vamorolone [who-dd]
19. Vbp15vbp15
20. Schembl143459
21. Gtpl9247
22. Amy7535
23. Dtxsid60927527
24. Bdbm50432396
25. Zinc33650317
26. Db15114
27. Pregna-1,4,9(11)-triene-3,20-dione, 17,21-dihydroxy-16-methyl-, (16.alpha.)-
28. Hy-109017
29. Cs-0030515
30. Dexamethasone Impurity E [ep Impurity]
31. D11000
32. 16.alpha.-methyl-9,11-dehydroprednisolone
33. J-006120
34. Q27089127
35. 17,21-dihydroxy-16.alpha.-methylpregna-1,4,9(11)-triene- 3,20-dione
36. 17.alpha.,21-dihydroxy-16.alpha.-methylpregna-1,4,9(11)-triene-3,20-dione
37. Delta-1,4,9(11)-pregnatriene-17-alpha,21-dihydroxy-16-alpha-methyl-3,20-dione
| Molecular Weight | 356.5 g/mol |
|---|---|
| Molecular Formula | C22H28O4 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 356.19875937 g/mol |
| Monoisotopic Mass | 356.19875937 g/mol |
| Topological Polar Surface Area | 74.6 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 775 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of Duchenne muscular dystrophy
Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
