
Synopsis
Synopsis
0
CEP/COS
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
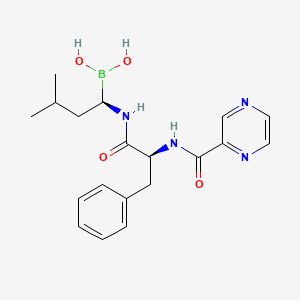

1. 341, Ps
2. Ldp 341
3. Ldp-341
4. Ldp341
5. Ps 341
6. Ps-341
7. Ps341
8. Velcade
1. 179324-69-7
2. Velcade
3. Ps-341
4. Ldp-341
5. Bortezomib (ps-341)
6. Radiciol
7. Ps 341
8. ((r)-3-methyl-1-((s)-3-phenyl-2-(pyrazine-2-carboxamido)propanamido)butyl)boronic Acid
9. Dpba
10. Ldp 341
11. Bortezomib Accord
12. Nsc-681239
13. [(1r)-3-methyl-1-[[(2s)-3-phenyl-2-(pyrazine-2-carbonylamino)propanoyl]amino]butyl]boronic Acid
14. Bortezomib (velcade)
15. N-[(1r)-1-(dihydroxyboryl)-3-methylbutyl]-n-(pyrazin-2-ylcarbonyl)-l-phenylalaninamide
16. Mln-341
17. [(1r)-3-methyl-1-[[(2s)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl)amino]propyl]amino]butyl]boronic Acid
18. Mg 341
19. Mg-341
20. Boronic Acid, B-[(1r)-3-methyl-1-[[(2s)-1-oxo-3-phenyl-2-[(2-pyrazinylcarbonyl)amino]propyl]amino]butyl]-
21. Nsc 681239
22. 69g8bd63pp
23. Chembl325041
24. Peptide Boronate
25. Chebi:52717
26. Nsc681239
27. Ncgc00242506-02
28. S1013
29. Dsstox_cid_20980
30. Dsstox_rid_79609
31. N-[(1r)-1-(dihydroxyboranyl)-3-methylbutyl]-nalpha-(pyrazin-2-ylcarbonyl)-l-phenylalaninamide
32. Dsstox_gsid_40980
33. Pyz-phe-boroleu
34. (r)-3-methyl-1-((s)-3-phenyl-2-(pyrazine-2-carboxamido)propanamido)butylboronic Acid
35. ((1r)-3-methyl-1-(((2s)-3-phenyl-2-((pyrazinylcarbonyl)amino)propanoyl)amino)butyl)boronic Acid
36. [(1r)-3-methyl-1-({(2s)-3-phenyl-2-[(pyrazin-2-ylcarbonyl)amino]propanoyl}amino)butyl]boronic Acid
37. Boronic Acid, ((1r)-3-methyl-1-(((2s)-1-oxo-3-phenyl-2-((pyrazinylcarbonyl)amino)propyl)amino)butyl)-
38. N-((1s)-1-benzyl-2-(((1r)-1-(dihydroxyboranyl)-3-methylbutyl)amino)-2-oxoethyl)pyrazinecarboxamide
39. Velcade (tn)
40. Cas-179324-69-7
41. Ps 341 (pharmaceutical)
42. Unii-69g8bd63pp
43. Brotezamide
44. Proscript Boronic Acid
45. Bortezomib [usan:inn:ban]
46. Hsdb 7666
47. Bortezomib Hydrate
48. Bortezomib,velcade
49. Lpd 341
50. Lpd-341
51. Ncgc00168751-01
52. Ncgc00181022-01
53. Bortezomib- Bio-x
54. Bortezomib [usan]
55. Mfcd09056737
56. Velcade (millenium)
57. 3mg0
58. Bortezomib [mi]
59. Bortezomib [inn]
60. Bortezomib [jan]
61. Bortezomib [hsdb]
62. Bortezomib [vandf]
63. Bortezomib [mart.]
64. Bortezomib [who-dd]
65. Mls004774142
66. Bortezomib (jan/usan/inn)
67. Bortezomib [ema Epar]
68. Schembl192129
69. Gtpl6391
70. Bortezomib [orange Book]
71. Bortezomib Hydrate [jan]
72. Dtxsid3040980
73. Tox21_112630
74. Tox21_112672
75. Bdbm50069989
76. Nsc756655
77. Akos015909706
78. Tox21_112672_1
79. Zinc169746649
80. Am81235
81. Ccg-268449
82. Cs-1039
83. Db00188
84. Nsc-756655
85. Bortezomib (velcade,mg-341,ps-341)
86. Ncgc00242506-01
87. Ncgc00242506-06
88. Ncgc00242506-07
89. As-15721
90. Bb164258
91. Hy-10227
92. Nci60_029010
93. Smr003500787
94. B5741
95. Sw208077-3
96. A18332
97. D03150
98. Ab01273951-01
99. Ab01273951-02
100. Ab01273951_03
101. 324b697
102. Q419319
103. Sr-01000939863
104. Sr-01000939863-2
105. Brd-k88510285-001-02-0
106. ((r)-3-methyl-1-((s)-3-phenyl-2-(pyrazine-2-carboxamido)propanamido)butyl)boronicacid
107. (r)-3-methyl-1-((s)-3-phenyl-2-(pyrazine-6-carboxamido)propanamido)butylboronic Acid
108. (1r)-3-methyl-1-({(2s)-3-phenyl-2-[(2-pyrazinylcarbonyl)amino]propanoyl}amino)butylboronic Acid, Aldrichcpr
109. (r)-3-methyl-1-((s)-3-phenyl-2-(pyrazine-2-carboxamido)propanamido)butan-2-ylboronic Acid
110. [(1r)-3-methyl-1-[[(2s)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl)amino]propyl]amino]-butyl]boronic Acid
111. 1610526-91-4
112. Boronic Acid, (3-methyl-1-((1-oxo-3-phenyl-2-((pyrazinylcarbonyl)amino)propyl)amino)butyl)-, (s-(r*,s*))-
113. Boronic Acid, [(1(r)-3-methyl-1-[[(2s)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl)amino]propyl]amino]butyl]-
114. N-((1s)-1-benzyl-2-(((1r)-1-(dihydroxyboranyl)-3-methylbutyl)amino)2-oxoethylpyrazinecarboxamide
115. N-[(1r)-1-(dihydroxyboranyl)-3-methylbutyl]-n(alpha)-(pyrazin-2-ylcarbonyl)-l-phenylalaninamide
| Molecular Weight | 384.2 g/mol |
|---|---|
| Molecular Formula | C19H25BN4O4 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 9 |
| Exact Mass | 384.1968855 g/mol |
| Monoisotopic Mass | 384.1968855 g/mol |
| Topological Polar Surface Area | 124 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 500 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Velcade |
| PubMed Health | Bortezomib (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | VELCADE (bortezomib) for Injection is an antineoplastic agent available for intravenous injection or subcutaneous use. Each single use vial contains 3.5 mg of bortezomib as a sterile lyophilized powder. Inactive ingredient: 35 mg mannitol, USP.Bort... |
| Active Ingredient | Bortezomib |
| Dosage Form | Injectable |
| Route | Intravenous, subcutaneous |
| Strength | 3.5mg/vial |
| Market Status | Prescription |
| Company | Millennium Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Velcade |
| PubMed Health | Bortezomib (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | VELCADE (bortezomib) for Injection is an antineoplastic agent available for intravenous injection or subcutaneous use. Each single use vial contains 3.5 mg of bortezomib as a sterile lyophilized powder. Inactive ingredient: 35 mg mannitol, USP.Bort... |
| Active Ingredient | Bortezomib |
| Dosage Form | Injectable |
| Route | Intravenous, subcutaneous |
| Strength | 3.5mg/vial |
| Market Status | Prescription |
| Company | Millennium Pharms |
Antineoplastic Agents; Protease Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Bortezomib injection is indicated for the treatment of patients with multiple myeloma who have received at least 1 prior therapy. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Velcade (Bortezomib) (January 2008). Available from, as of November 25, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6358
Bortezomib injection is indicated for the treatment of patients with mantle cell lymphoma who have received at least 1 prior therapy. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Velcade (Bortezomib) (January 2008). Available from, as of November 25, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6358
Known hypersensitivity to bortezomib, boron, or mannitol.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 929
Bortezomib mainly causes sensory peripheral neuropathy, but severe motor peripheral neuropathy also has been reported. In the phase III trial, peripheral neuropathy occurred in 36% of patients receiving bortezomib and 9% of patients receiving dexamethasone. Grade 3 or 4 peripheral neuropathy occurred in 7 or less than 1%, respectively, of patients receiving bortezomib. Following dosage adjustments, amelioration or resolution of peripheral neuropathy was reported in 51% of patients with grade 2 or higher peripheral neuropathy within a median of 3.5 months from onset. About 8% of patients discontinued bortezomib therapy because of peripheral neuropathy.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 929
Patients receiving bortezomib should be monitored for manifestations of neuropathy (eg, burning sensation, hyperesthesia, hypoesthesia, paresthesia, discomfort, neuropathic pain). Dose and/or frequency of administration of bortezomib should be adjusted in patients who experience new-onset or exacerbation of peripheral neuropathy.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 929
In the phase III trial, asthenia (ie, fatigue, malaise, weakness) was reported in 61% of patients receiving bortezomib and 45% of patients receiving dexamethasone. Grade 3 asthenia occurred in 12 versus 6%, respectively, of patients receiving bortezomib or dexamethasone. About 3% of patients receiving bortezomib and 2% of patients receiving dexamethasone discontinued therapy because of asthenia.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 929
For more Drug Warnings (Complete) data for BORTEZOMIB (26 total), please visit the HSDB record page.
Bortezomib is indicated for the treatment of adult patients with multiple myeloma or mantle cell lymphoma.
FDA Label
Velcade as monotherapy or in combination with pegylated liposomal doxorubicin or dexamethasone is indicated for the treatment of adult patients with progressive multiple myeloma who have received at least 1 prior therapy and who have already undergone or are unsuitable for haematopoietic stem cell transplantation.
Velcade in combination with melphalan and prednisone is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for high dose chemotherapy with haematopoietic stem cell transplantation.
Velcade in combination with dexamethasone, or with dexamethasone and thalidomide, is indicated for the induction treatment of adult patients with previously untreated multiple myeloma who are eligible for high dose chemotherapy with haematopoietic stem cell transplantation.
Velcade in combination with rituximab, cyclophosphamide, doxorubicin and prednisone is indicated for the treatment of adult patients with previously untreated mantle cell lymphoma who are unsuitable for haematopoietic stem cell transplantation.
Bortezomib Hospira as monotherapy or in combination with pegylated liposomal doxorubicin or dexamethasone is indicated for the treatment of adult patients with progressive multiple myeloma who have received at least 1 prior therapy and who have already undergone or are unsuitable for haematopoietic stem cell transplantation.
Bortezomib Hospira in combination with melphalan and prednisone is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for high-dose chemotherapy with haematopoietic stem cell transplantation.
Bortezomib Hospira in combination with dexamethasone, or with dexamethasone and thalidomide, is indicated for the induction treatment of adult patients with previously untreated multiple myeloma who are eligible for high-dose chemotherapy with haematopoietic stem cell transplantation.
Bortezomib Hospira in combination with rituximab, cyclophosphamide, doxorubicin and prednisone is indicated for the treatment of adult patients with previously untreated mantle cell lymphoma who are unsuitable for haematopoietic stem cell transplantation.
Bortezomib as monotherapy or in combination with pegylated liposomal doxorubicin or dexamethasone is indicated for the treatment of adult patients with progressive multiple myeloma who have received at least 1 prior therapy and who have already undergone or are unsuitable for haematopoietic stem cell transplantation.
Bortezomib in combination with melphalan and prednisone is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for high-dose chemotherapy with haematopoietic stem cell transplantation.
Bortezomib in combination with dexamethasone, or with dexamethasone and thalidomide, is indicated for the induction treatment of adult patients with previously untreated multiple myeloma who are eligible for high-dose chemotherapy with haematopoietic stem cell transplantation.
Bortezomib in combination with rituximab, cyclophosphamide, doxorubicin and prednisone is indicated for the treatment of adult patients with previously untreated mantle cell lymphoma who are unsuitable for haematopoietic stem cell transplantation.
Bortezomib SUN as monotherapy or in combination with pegylated liposomal doxorubicin or dexamethasone is indicated for the treatment of adult patients with progressive multiple myeloma who have received at least 1 prior therapy and who have already undergone or are unsuitable for haematopoietic stem cell transplantation.
Bortezomib SUN in combination with melphalan and prednisone is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for high-dose chemotherapy with haematopoietic stem cell transplantation.
Bortezomib SUN in combination with dexamethasone, or with dexamethasone and thalidomide, is indicated for the induction treatment of adult patients with previously untreated multiple myeloma who are eligible for high-dose chemotherapy with haematopoietic stem cell transplantation.
Bortezomib SUN in combination with rituximab, cyclophosphamide, doxorubicin and prednisone is indicated for the treatment of adult patients with previously untreated mantle cell lymphoma who are unsuitable for haematopoietic stem cell transplantation.
Bortezomib Accord as monotherapy or in combination with pegylated liposomal doxorubicin or dexamethasone is indicated for the treatment of adult patients with progressive multiple myeloma who have received at least 1 prior therapy and who have already undergone or are unsuitable for haematopoietic stem cell transplantation.
Bortezomib Accord in combination with melphalan and prednisone is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for highdose chemotherapy with haematopoietic stem cell transplantation.
Bortezomib Accord in combination with dexamethasone, or with dexamethasone and thalidomide, is indicated for the induction treatment of adult patients with previously untreated multiple myeloma who are eligible for highdose chemotherapy with haematopoietic stem cell transplantation.
Bortezomib Accord in combination with rituximab, cyclophosphamide, doxorubicin and prednisone is indicated for the treatment of adult patients with previously untreated mantle cell lymphoma who are unsuitable for haematopoietic stem cell transplantation.
Treatment of mantle cell lymphoma
Bortezomib works to target the ubiquitin-proteasome pathway, an essential molecular pathway that regulates intracellular concentrations of proteins and promotes protein degradation. The ubiquitin-proteasome pathway is often dysregulated in pathological conditions, leading to aberrant pathway signalling and the formation of malignant cells. In one study, patient-derived chronic lymphocytic leukemia (CLL) cells contained 3-fold higher levels of chymotrypsin-like proteasome activity than normal lymphocytes. By reversibly inhibiting proteasome, bortezomib prevents proteasome-mediated proteolysis. Bortezomib exerts a cytotoxic effect on various cancer cell types _in vitro_ and delays tumour growth _in vivo_ in nonclinical tumour models. Bortezomib inhibits the proteasome activity in a dose-dependent manner. In one pharmacodynamic study, more than 75% of proteasome inhibition was observed in whole blood samples within one hour after dosing of bortezomib.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
L01XG01
L01XG01
L01XG01
L01XG01
L01XX32
L01XX32
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01X - Other antineoplastic agents
L01XG - Proteasome inhibitors
L01XG01 - Bortezomib
Absorption
Following intravenous administration of 1 mg/m2 and 1.3 mg/m2 doses, the mean Cmax of bortezomib were 57 and 112 ng/mL, respectively. In a twice-weekly dosing regimen, the Cmax ranged from 67 to 106 ng/mL at the dose of 1 mg/m2 and 89 to 120 ng/mL for the 1.3 mg/m2 dose. In patients with multiple myeloma, the Cmax of bortezomib followig subcutaneous administration was lower than that of intravenously-administered dose; however, the total systemic exposure of the drug was equivalent for both routes of administration. There is a wide interpatient variability in drug plasma concentrations.
Route of Elimination
Bortezomib is eliminated by both renal and hepatic routes.
Volume of Distribution
The mean distribution volume of bortezomib ranged from approximately 498 to 1884 L/m2 in patients with multiple myeloma receiving a single- or repeat-dose of 1 mg/m2 or 1.3 mg/m2. Bortezomib distributes into nearly all tissues, except for the adipose and brain tissue.
Clearance
Following the administration of a first dose of 1 mg/m2 and 1.3 mg/m2, the mean mean total body clearances were 102 and 112 L/h, respectively. The clearances were 15 and 32 L/h after the subsequent dose of 1 and 1.3 mg/m2, respectively.
Following intravenous administration of 1 mg/sq m and 1.3 mg/sq m doses to 24 patients with multiple myeloma (n=12, per each dose level), the mean maximum plasma concentrations of bortezomib (Cmax) after the first dose (Day 1) were 57 and 112 ng/mL, respectively.
US Natl Inst Health; DailyMed. Current Medication Information for Velcade (Bortezomib) (January 2008). Available from, as of November 25, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6358
In subsequent doses, when administered twice weekly, the mean maximum observed plasma concentrations ranged from 67 to 106 ng/mL for the 1 mg/sq m dose and 89 to 120 ng/mL for the 1.3 mg/sq m dose. The mean elimination half-life of bortezomib upon multiple dosing ranged from 40 to 193 hours after the 1 mg/sq m dose and 76 to 108 hours after the 1.3 mg/sq m dose. The mean total body clearances was 102 and 112 L/hr following the first dose for doses of 1 mg/sq m and 1.3 mg/sq m, respectively, and ranged from 15 to 32 L/hr following subsequent doses for doses of 1 and 1.3 mg/sq m, respectively.
US Natl Inst Health; DailyMed. Current Medication Information for Velcade (Bortezomib) (January 2008). Available from, as of November 25, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6358
The mean distribution volume of bortezomib ranged from approximately 498 to 1884 L/sq m following single- or repeat-dose administration of 1 mg/sq m or 1.3mg/sq m to patients with multiple myeloma. This suggests bortezomib distributes widely to peripheral tissues. The binding of bortezomib to human plasma proteins averaged 83% over the concentration range of 100 to 1000 ng/mL.
US Natl Inst Health; DailyMed. Current Medication Information for Velcade (Bortezomib) (January 2008). Available from, as of November 25, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6358
It is not known whether bortezomib is excreted in human milk.
US Natl Inst Health; DailyMed. Current Medication Information for Velcade (Bortezomib) (January 2008). Available from, as of November 25, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6358
For more Absorption, Distribution and Excretion (Complete) data for BORTEZOMIB (6 total), please visit the HSDB record page.
Bortezomib is primarily metabolized by CYP3A4, CYP2C19, and CYP1A2. CYP2D6 and CYP2C9 are also involved in drug metabolism, but to a smaller extent. Oxidative deboronation, which involves the removal of boronic acid from the parent compound, is the main metabolic pathway. Metabolites of bortezomib are pharmacologically inactive and more than 30 metabolites have been identified in human and animal studies.
In vitro studies with human liver microsomes and human cDNA-expressed cytochrome P450 isozymes indicate that bortezomib is primarily oxidatively metabolized via cytochrome P450 enzymes 3A4, 2C19, and 1A2. Bortezomib metabolism by CYP 2D6 and 2C9 enzymes is minor. The major metabolic pathway is deboronation to form 2 deboronated metabolites that subsequently undergo hydroxylation to several metabolites. Deboronated bortezomib metabolites are inactive as 26S proteasome inhibitors. Pooled plasma data from 8 patients at 10 min and 30 min after dosing indicate that the plasma levels of metabolites are low compared to the parent drug.
US Natl Inst Health; DailyMed. Current Medication Information for Velcade (Bortezomib) (January 2008). Available from, as of November 25, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6358
... The P450 inhibition potential of bortezomib and its major deboronated metabolites M1 and M2 and their dealkylated metabolites M3 and M4 was evaluated in human liver microsomes for the major P450 isoforms 1A2, 2C9, 2C19, 2D6, and 3A4/5. Bortezomib, M1, and M2 were found to be mild inhibitors of CYP2C19 (IC(50) approximately 18.0, 10.0, and 13.2 microM, respectively), and M1 was also a mild inhibitor of CYP2C9 (IC(50) approximately 11.5 microM). However, bortezomib, M1, M2, M3, and M4 did not inhibit other P450s (IC(50) values > 30 microM). There also was no time-dependent inhibition of CYP3A4/5 by bortezomib or its major metabolites. ...
PMID:16443666 LU C et al; Drug Metab Dispos 34 (4): 702-8 (2006)
... Bortezomib binds the proteasome via the boronic acid moiety, and therefore, the presence of this moiety is necessary to achieve proteasome inhibition. Metabolites in plasma obtained from patients receiving a single intravenous dose of bortezomib were identified and characterized by liquid chromatography/mass spectrometry (LC/MS) and liquid chromatography/tandem mass spectrometry (LC/MS/MS). Metabolite standards that were synthesized and characterized by LC/MS/MS and high field nuclear magnetic resonance spectroscopy (NMR) were used to confirm metabolite structures. The principal biotransformation pathway observed was oxidative deboronation, most notably to a pair of diastereomeric carbinolamide metabolites. Further metabolism of the leucine and phenylalanine moieties produced tertiary hydroxylated metabolites and a metabolite hydroxylated at the benzylic position, respectively. Conversion of the carbinolamides to the corresponding amide and carboxylic acid was also observed. Human liver microsomes adequately modeled the in vivo metabolism of bortezomib, as the principal circulating metabolites were observed in vitro. Using cDNA-expressed cytochrome P450 isoenzymes, it was determined that several isoforms contributed to the metabolism of bortezomib, including CYP3A4, CYP2C19, CYP1A2, CYP2D6, and CYP2C9. ...
PMID:15764713 Pekol T et al; Drug Metab Dispos 33 (6): 771-7 (2005)
The mean elimination half-life of bortezomib ranged from 40 to 193 hours following a multiple dosing regimen at a 1 mg/m2 dose. The half-life ranged from 76 to 108 hours after multiple dosing of 1.3 mg/m2 bortezomib.
The mean elimination half-life of bortezomib upon multiple dosing ranged from 40 to 193 hours after the 1 mg/sq m dose and 76 to 108 hours after the 1.3 mg/sq m dose.
US Natl Inst Health; DailyMed. Current Medication Information for Velcade (Bortezomib) (January 2008). Available from, as of November 25, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6358
The ubiquitin-proteasome pathway is a homeostatic proteolytic pathway for intracellular protein degradation: proteins marked with a poly-ubiquitin chain are degraded to small peptides and free ubiquitin by the proteasome, which is a large multimeric protease. Aberrant proteasome-dependent proteolysis, as seen in some malignancies, can lead to uncontrolled cell division, leading to tumorigenesis, cancer growth, and spread. Bortezomib is a reversible inhibitor of the 26S proteasome, which is made up of a 20S core complexed with a 19S regulatory complex. Individual -subunits allow specific catalytic action of the 20S core. In mammalian cells, bortezomib is a potent inhibitor of the proteasomes chymotryptic-like activity, which is attributed to the 5-subunit of the 20S core particle. Bortezomib binds to the active site of the threonine hydroxyl group in the 5-subunit. A probing study showed bortezomib also binding to and inhibiting the 1-subunit, which mediates the caspase-like activity of the proteasome, and 1i-subunit, which is an altered subunit that is expressed to form immunoproteasomes in response to cell stress or inflammation. By inhibiting the proteasome-mediated degradation of key proteins that promote cell apoptosis, bortezomib induces a cell cycle arrest during the G2-M phase. It is believed that multiple mechanisms, other than proteasome inhibition, may be involved in the anticancer activity of bortezomib. The anticancer activity of bortezomib was largely associated with suppression of the NF-B signalling pathway, resulting in the downregulation of anti-apoptotic target genes and expression of anti-apoptic proteins. This may be explained by bortezomib preventing uncontrolled degradation of IB, which is an inhibitory protein of NF-B. NOXA, which is a pro-apoptotic factor, induced by bortezomib selectively in cancer cells; thus, it is suggested to be another key mechanism of bortezomib.
Bortezomib, a modified dipeptidyl boronic acid, is an antineoplastic agent. The drug reversibly inhibits the 26S proteasome, a large protein complex that degrades ubiquitinated proteins. The ubiquitin-proteasome pathway plays an essential role in regulating the intracellular concentration of specific proteins, thereby maintaining homeostasis within cells. Inhibition of the 26S proteasome by bortezomib prevents targeted proteolysis and causes disruption of normal homeostatic mechanisms, which can lead to cell death. In vitro studies indicate that bortezomib is cytotoxic to a variety of cancer cell types. Bortezomib has been shown to cause a delay in tumor growth in vivo in tumor models, including multiple myeloma.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 931
... The mechanisms underlying /bortezomib/ cancer cell toxicity are complex. A growing body of evidence suggests proteasome inhibition-dependent regulation of the BCL-2 family is a critical requirement. In particular, the stabilization of BH3-only proteins BIK, NOXA and BIM, appear to be essential for effecting BAX- and BAK-dependent cell death. ...
PMID:17828309 Fennell DA et al; Oncogene 27 (9): 1189-97 (2008)
Proteasome inhibition is a novel, targeted approach in cancer therapy. Both natural and synthetic proteasome inhibitors selectively penetrate cancer cells, disrupting the orderly destruction of key regulatory proteins involved in tumorigenesis and metastasis. Disrupting the orderly destruction of regulatory proteins causes an imbalance of these proteins within the cell, which interferes with the systematic activation of signaling pathways required to maintain tumor cell growth and survival; therefore, cellular replication is inhibited and apoptosis ensues. ...
PMID:15688597 Jung L et al; Oncology (Williston Park) 18 (14 Suppl 11): 4-13 (2004)
Bortezomib (PS-341, Velcade) is a potent and selective inhibitor of the proteasome that is currently under investigation for the treatment of solid malignancies. /Investigators/ have shown previously that bortezomib has activity in pancreatic cancer models and that the drug induces endoplasmic reticulum (ER) stress but also suppresses the unfolded protein response (UPR). Because the UPR is an important cytoprotective mechanism, /investigators/ hypothesized that bortezomib would sensitize pancreatic cancer cells to ER stress-mediated apoptosis. Here, /the authors/ show that bortezomib promotes apoptosis triggered by classic ER stress inducers (tunicamycin and thapsigargin) via a c-Jun NH(2)-terminal kinase (JNK)-dependent mechanism. /They/ also show that cisplatin stimulates ER stress and interacts with bortezomib to increase ER dilation, intracellular Ca(2+) levels, and cell death. Importantly, combined therapy with bortezomib plus cisplatin induced JNK activation and apoptosis in orthotopic pancreatic tumors resulting in a reduction in tumor burden. Taken together, the data establish that bortezomib sensitizes pancreatic cancer cells to ER stress-induced apoptosis and show that bortezomib strongly enhances the anticancer activity of cisplatin.
PMID:16357177 Nawrocki ST et al; Cancer Res 65 (24): 11658-66 (2005)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
24
PharmaCompass offers a list of Bortezomib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Bortezomib manufacturer or Bortezomib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Bortezomib manufacturer or Bortezomib supplier.
PharmaCompass also assists you with knowing the Bortezomib API Price utilized in the formulation of products. Bortezomib API Price is not always fixed or binding as the Bortezomib Price is obtained through a variety of data sources. The Bortezomib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Velcade manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Velcade, including repackagers and relabelers. The FDA regulates Velcade manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Velcade API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Velcade manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Velcade supplier is an individual or a company that provides Velcade active pharmaceutical ingredient (API) or Velcade finished formulations upon request. The Velcade suppliers may include Velcade API manufacturers, exporters, distributors and traders.
click here to find a list of Velcade suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Velcade DMF (Drug Master File) is a document detailing the whole manufacturing process of Velcade active pharmaceutical ingredient (API) in detail. Different forms of Velcade DMFs exist exist since differing nations have different regulations, such as Velcade USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Velcade DMF submitted to regulatory agencies in the US is known as a USDMF. Velcade USDMF includes data on Velcade's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Velcade USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Velcade suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Velcade Drug Master File in Japan (Velcade JDMF) empowers Velcade API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Velcade JDMF during the approval evaluation for pharmaceutical products. At the time of Velcade JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Velcade suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Velcade Drug Master File in Korea (Velcade KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Velcade. The MFDS reviews the Velcade KDMF as part of the drug registration process and uses the information provided in the Velcade KDMF to evaluate the safety and efficacy of the drug.
After submitting a Velcade KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Velcade API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Velcade suppliers with KDMF on PharmaCompass.
A Velcade written confirmation (Velcade WC) is an official document issued by a regulatory agency to a Velcade manufacturer, verifying that the manufacturing facility of a Velcade active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Velcade APIs or Velcade finished pharmaceutical products to another nation, regulatory agencies frequently require a Velcade WC (written confirmation) as part of the regulatory process.
click here to find a list of Velcade suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Velcade as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Velcade API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Velcade as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Velcade and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Velcade NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Velcade suppliers with NDC on PharmaCompass.
Velcade Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Velcade GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Velcade GMP manufacturer or Velcade GMP API supplier for your needs.
A Velcade CoA (Certificate of Analysis) is a formal document that attests to Velcade's compliance with Velcade specifications and serves as a tool for batch-level quality control.
Velcade CoA mostly includes findings from lab analyses of a specific batch. For each Velcade CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Velcade may be tested according to a variety of international standards, such as European Pharmacopoeia (Velcade EP), Velcade JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Velcade USP).
